

Romix
-
Posts
23 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Romix
-
-
What about boiling it on low flame.
And when all dissolve, dilute it with distill water, would Sn and Pb oxides precipitate out?
0 -
Ok, if I boil boards in NaOH.
All the solder should dissolve in it.
Forming Na2[sn(OH)6] and Na2[Pb(OH)4]
Na2[sn(OH)6] will decompose to Na2SnO3 + 3H2O
And I'm shore it will! Last time I heated aluminum chloride on my hotplate, it decomposed it, and temperatures for AlCl3 are much higher.
Na2[Pb(OH)4] decomposes to Na2PbO3 at the temperature of 300°C, about the same as for AlCl3.
I left with solution of Na2SnO3, Na2PbO3 and NaOH?
0 -
Why bases sucks moisture from the air and melts?
0 -
At that temperature aluminium oxide will dissolve in cryolite.
If you pass an electric current through the solution you will get aluminium metal and oxygen (which will probably attack the electrode).
Yes agree, if electrodes were made of carbon, platinum electrodes will be fine.
What actually happens to the molecules when cryolite used as solvent to dissolve aluminium oxide?
For H2O and NaCl google gave me this picture.
Not shore if its right.
Here is anion and cation of NaCl in solution of H2O.

Still don't get it, why dissolution happening? Why some salts are insoluble.
Is it something to do with their electron configuration?
Alright lets start from simple insoluble ionic metal salts.
Barium Sulphate
Lead Chloride and Sulphate
Silver Chloride
That's the electron configuration of metals.
Silver

Barium

Lead

Anion:
SO4 with a -2 charge and Cl with a -1 charge.
Silver transfers one electron to chlorine atom. Usually it happening in displacement reaction, but can be done directly with addition of H2O2.
Ok no electrons left in its 5th outer shell, and chlorine filled its shells.
Strong ionic bond.
Same situation I see in bonding Sodium with Chlorine.
But salt produced is soluble.
Maybe because Sodium is Alkali Metal, and its electrons really close to the nucleus?
Barium Sulphate another insoluble salt.
Barium have 2 electrons in its outer shell.
[ba+2][sO4-2]
All its shells filled.
This time anion not just atom, its a molecule.
Lead Chloride & Sulphate are both insoluble.
4 electrons in its outer shell...
Why this salts are insoluble?
0 -
What happening to aluminium oxide in molten cryolite (Na3AlF6) at 1000°C ?
0 -
Why Hydrogen and Chlorine reacts so violently under UV lights forming a bond?

How many moles of HCl need to be produced to explode the 800 ml beaker covering beaker with solute of NaCl in to shards?
0 -
You should look up a bond enthalpy table if you haven't already been given one.
I have book, but C-C bond not in there.
I googled, enthalpy table and found one.
Can you please check if the numbers in it are right?
 0
0 -
How much kj mol-1 needed to brake bond between two Carbons in Heptane?
0 -
How to separate Sodium ion from Stannous Hydroxide ion?
By boiling it in H2O?

Why stannouses valency in this molecule is (VI) ?
0 -
Why FeCl3 is(III) and FeI (II) ?
0 -
If you don't want insulation to dissolve, and contaminate solution, do the same as in Hofmann's voltameter
http://en.wikipedia.org/wiki/Hofmann_voltameter
Place electrodes up-side-down in bottom of container.
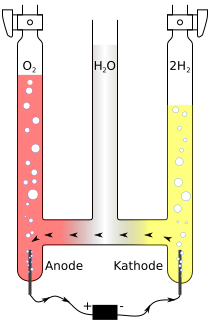
You can buy ready device for 50 ukp, see f.e.
http://www.sci-mart.com/Chemistry/Stands/hoffman-voltmeter
Thanks for link, ordered graphite rods there.
0 -
There is an urban legend about "bright orange THC worms", which looking at the pictures/descriptions look like wireworms(actually a type of beetle larvae). Supposedly they concentrate THC. As one might expect from a pot centric urban legend the different tales vary on whether you need to smoke their poop or little worm bodies to get this effect.
Onwards to Earthworms...
Somewhat ironically, in searching, I found that one of my "go-to" sites already did an article on them: http://www.eattheweeds.com/cooking-with-earthworms-2/
No word on if smoking them will get you high, but if you want to eat them feel free(after proper food preparation).
Im talking about Bananadine
0 -
If I feed them with bannanas in one bag and apple's in another.
And my friends from Holland joined this experiment, he feeds his with canni leafs.
What will happen to strong bond alkaloids inside worm?
And what if I feed them worms to ill in a fish-tanks.
How it will change the molecule?
And what if I bake this ills later and roll them in to sushi and eat it.
Would they get me high?
Where molecule goes next pee or poo?
All wrong, extracting from ill poo not a good idea !
0 -
I need NaOH for one experiment, by doing this I kill two rabbits in one shot.
0 -
Can solution of NaOH dissolve isolation rubber around copper wire?

Would heated alloy of lead and tin react with Cl2(g)?
If they do react, anhydrous PbCl2 and anhydrous SnCl4 will form.
Dissolve them in still H2O, SnCl4 soluble and PbCl2 not.
Filter of insoluble Lead(II) Chloride and wash it.
After pure tin crystals can be recovered by electrolyzing solution what's left.
0 -
Potassium
Sodium
Lithium
Magnesium
Aluminum
Titanium
Manganese
Zinc
Iron
Cobalt
Chromium
Nickel
Tin
Lead
Copper
Silver
Gold
Palladium
Tantalum
Ruthenium
Iodine
Sulphur
Silicon
Chlorine
Hydrogen
Oxygen
0 -
Can H2 and Cl2 gases be bonded together to form 2Hcl and how?
With out explosion please.
0 -
Ok, thanks
0 -
Hello dear forum members, here's the question for you.
CuSO4 + 5H2O ---> CuSO4.5H20
Where this 5 came from?
How u work out that it's 5?
Na2SO4 + 10H20 ---> Na2SO4.10H20
Same question here, why there's ten H2O?
0 -


like this ?
Ok all Oxygens are happy, they have full outer valence shells of 8 electrons.
But chromium have 12 now! Something not right here.
0 -
oooo that's my mistake I counted chromium as (V) and it's (VI)
0 -
Hello all.
Can some one expain me please how this double bonds are formed?

 0
0

Dissolution of NaOH in H2O
in Inorganic Chemistry
Posted
Dissolution of NaOH in H2O
Why this reaction is extorhermic?