

Matonis
-
Posts
11 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Matonis
-
-
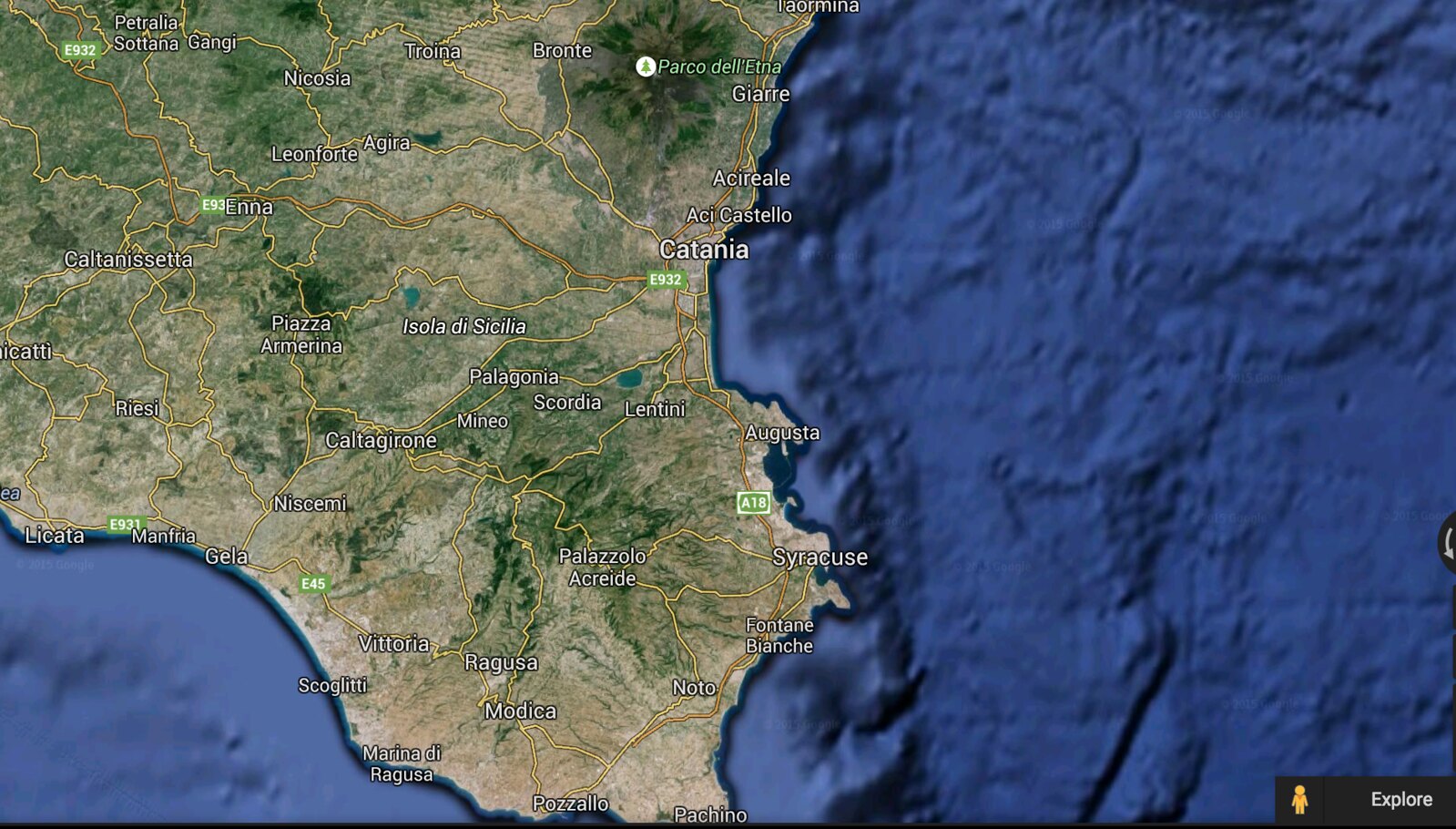
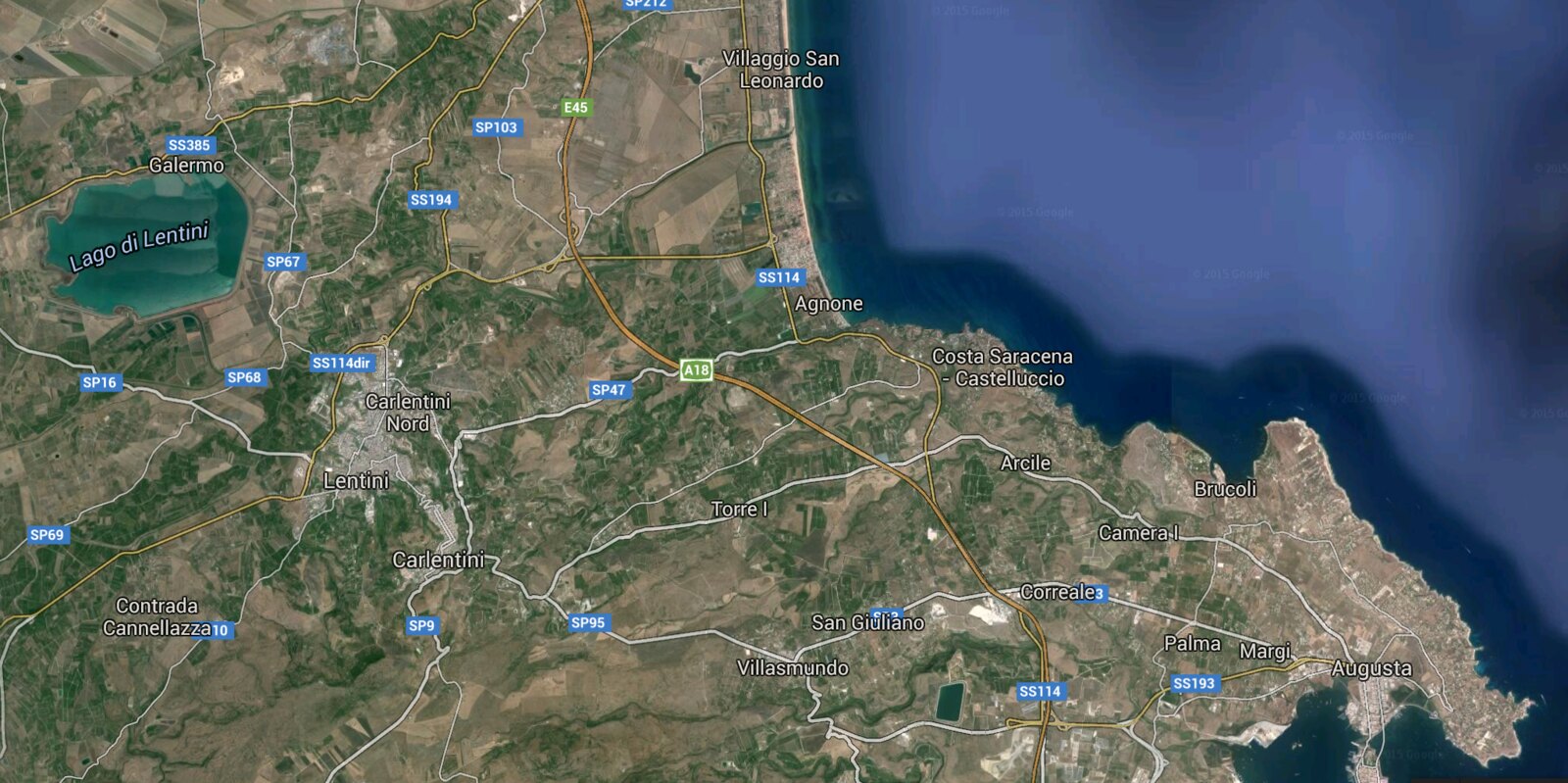
To be more specific, the artifact was found roughly 15 minutes south of Agnone, Sicily. Pictures below.
Also a link to images of Ancient Sicilian Amphora
0 -
About 10 years ago I went to Sicily and during my stay there I explored a limestone canyon (not sure what it would be classified as). I had a great time exploring small caves and used chisels and brushes to excavate some small fossils. This was not a heavily developed area, virtually no tourists, and the site was on private property (I was given permission by the property owner).
While searching for fossils, I found (what appears to be) a vase handle and have kept it ever since. My question is...how old is it? I'm not trying to scam anyone or anything but, should this be in a museum?
Is it too "young" to carbon date? Is it likely just a piece of trash from 20 years ago or is it a relic from...1000 years ago? (idk)
Thank you for your help



 0
0 -
Using a patented process I have a tiny amount of dried solubilized keratin, which supposedly can then be used to create fibers.
My question is how to preform the later step.
In basic terms I broke hair down, took out the keratin, added some "stuff" to it, solubilized and dried it, and now I'm trying to restore it back to fiber form; or at the very least, something more solid than powder.
Any help would be much appreciated.
- 0
0 -
Using a patented process I have a tiny amount of dried solubilized keratin, which supposedly can then be used to create fibers.
My question is how to preform the later step.
In basic terms I broke hair down, took out the keratin, added some "stuff" to it, solubilized and dried it, and now I'm trying to restore it back to fiber form; or at the very least, something more solid than powder.
Any help would be much appreciated.
0 -
I essentially need to dissolve a substance with a high percentage of keratin.
The tricky, counter intuitive, conundrum is dissolving something without drastically altering its molecular structure.
This is because the substance/liquid will then be "re-polymerized" back into a solid filament.
FTIR scan practically identical to hair. We are using FTIR to basically take a before dissolving and after dissolving shot, if they look similar we can move on to the next step.
Thank you in advance for any responses or contributions, I appreciate it.
-S
http://www.google.com/patents/US2542984
This could work...maybe
0 -
Let's imagine that your material is a natural fibre with better mechanical properties than silk...
I vaguely suppose that its properties result from the synchronization of the peptides among the parallel macromolecules that make the fibre, within a cross section, and not from a particular sequence of the peptides within one macromolecule. After all, the "adhesion" between the macromolecules defines a fiber's strength better than the macromolecule alone does. (And the strength of a rope results very partially from the fiber)
Then, the unexplained strength of the not-so-mysterious fibre would result from the way it's produced by the organism, not from its detailed composition, whose peptide sequence can be largely random.
"You can't" melt and extrude a fibre, but exactly that would be useful! Because if my explanation attempt hold, this would ruin the fiber's strength even if the composition is kept in detail.
----------
From the time when I'm a mechanical designer, I'd like to stress that of course, I need ropes that are stronger, stiffer, lighter, and resist UV and water... First, man-made fibers already outperform all natural ones (yes, all). But even more, I need materials with unusual properties!
Mankind needs fibers that accept a big elongation, restore
I may be of assistance in the future with the a elastic fiber that regains its yield point after a short period of time.
I agree with your assessment on rope strength, that the strength is derived largely from the friction as opposed to the fibers themselves.
So, any suggestions on breaking the fibre down? I have ideas on building it back up, the difficulty is dissolving it without completely throwing off the chemistry for when I "re-make" it.
0 -
Radical polymerization is one of the standards of polymers. A radical is introduced and the bond is made generating a successive radical. Exothermal/endothermal considerations should be taken into account. Peroxide is a good one due to the presence of oxygen in the atmosphere. There is also the potential to polymerize with UV irradiation.
You're saying similar to hair. To "re"-polymerize something I would consider electro-chemistry. Sulfate ions have a strong electrical potential. SO4-2 + 2 e-> S2O8-2 is a reaction I have long been interested in seeing put to use. I think this is the same concept as the hair straightener. If sulfate could then be precipitated out, the repolymerization would be done with either a catalyst or hydrogen peroxide at your convenience. In theory, you could plate out the results. Sulfur requires platinum electrodes. But you can also dissolve an electrode deliberately.
BTW it would take a few days to a week to breakdown probably.
I think MSM, Methylsulfonylmethane, could have solvency interactions.
First off, thank you.
Secondly, which of these processes deals with breaking the substance down? Should I conceptualize this process more as swapping in and out polymer parts, as opposed to breaking down?
I will talk with a colleague while I wait for your answer, I will keep you updated with what I end up doing.
0 -
I have a Block Co-Polymer that is a type of fiber with an FTIR spectrum very similar to hair.
I need to some how break down a fiber, and synthesize it. If I could, which I can't, this could mean simply melting it and pouring the molten fiber into a mold. What are some methods that you can suggest I try? I feel that dialysis could be a option (spin in tubing, against acid or acetate based solution in hopes that something permeates through). I need to choose this route as opposed to artificial synthesizing because the material I'm looking at is incredibly complex to the point where mixing and chaining peptides would be very expensive (no one has successfully taken something like a synergy instrument and re-created it). I have FTIR scans and a decent amount of samples so I know what the end product should look like.
Properly re-assembling a complex molecular structure that has been broken down is what we're discussing.
Hair thinner?
I really need some 3rd party input, this is something new to me and there could be a very easy solution. I originally put this in the BioChem & Molecular Biology section but it hasn't gotten any replies, the chemistry forum looks to be where the parties are at...
Thank you.
0 -
I have a Block Co-Polymer that is a type of fiber with an FTIR spectrum very similar to hair.
I need to some how break down a fiber, and synthesize it. If I could, which I can't, this could mean simply melting it and pouring the molten fiber into a mold. What are some methods that you can suggest I try? I feel that dialysis could be a option (spin in tubing, against acid or acetate based solution in hopes that something permeates through). I need to choose this route as opposed to artificial synthesizing because the material I'm looking at is incredibly complex to the point where mixing and chaining peptides would be very expensive (no one has successfully taken something like a synergy instrument and re-created it). I have FTIR scans and a decent amount of samples so I know what the end product should look like.
Properly re-assembling a complex molecular structure that has been broken down is what we're discussing.
Hair thinner?
I really need some 3rd party input, this is something new to me and there could be a very easy solution.
Thank you.
0

Archaeological Question(s) on a Artifact I've Kept
in Earth Science
Posted
Guys...I'm not labelling this the find of a century lol. Just would be cool to know. To be able to say...hey it turns out this is _____ years old.
I'm looking for TL dating resources.