This thread was inspired by the following comment in this query about air conditioning.
But is that so ?
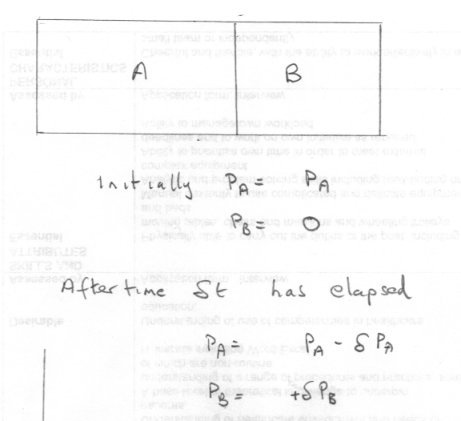
The change in entropy going from state A to state B is always the same, irrespective of the path between A and B since entropy is a state variable and thus a function of the state of the system alone.
It make no difference whether that path is reversible or irreversible.
Only in the case of a reversible path is the entropy given by the expression
[math]\Delta S