UC
Senior Members-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by UC
-
You're responding to a 3-years dead thread ressurected by spam.
-
Facetious. Water just happens to absorb weakly in the red region of visible light. Heavy water (Deuteurium oxide) is completely colorless when pure. The change in isotopes has shifted it's absorption band further into the IR spectrum. Now dissolve some alkali metals in liquid anhydrous ammonia for me, and you can see free electrons.
-
Google is your friend. http://en.wikipedia.org/wiki/PH_indicator Phenolphthalein is probably the most common in science labs. Bromothymol blue is also popular, and obviously Litmus.
-
HSbF6 decompses with much violence in water, hydrolyzing to HF and antimony oxides. 10^19 times stronger just means that the proton is 10^19 less strongly attached to the rest of the molecule. pH relies on concentration of protons (hydronium ions) in water, and will never exceed the -log(conc), where conc is the molarity of the acid in solution multiplied by the number of acidic protons. In water, any acid with a pKa lower than -1.7 is effectively completely dissosciated, forming a "salt" if you will of hydronium ion (analagous to ammonium) How corossive acids are doesn't mean anything with regards to their strength. HSbF6 needs to be handled with all fluoropolymer equipment, simply because the fluoride is immensely corrosive to glass.
-
Mercury vapor. While it may not conduct any better than normal gas, once an arc is struck, the plasma is an excellent conductor. For a particularly awesome looking piece of equipment, google mercury arc rectifier. As for nomenclature, the gas may not participate in metallic bonding anymore, but it is still of a 0-valent metallic element.
-
A good listing may or may not exist, but I haven't found one yet. Take some pics and I can give it a run-through and try to tell you what's what. Often there are bits of custom glass kicking around, which are anyone's guess.
-
At temperatures where magnesium is molten or burning, it's not unreasonable to expect hot, magnesium vapor to fill the crucible rapidly. I suspect that with the sudden influx of oxygen, it burned very rapidly.
-
Generally you'd use a heap of manure and other decaying matter, that is kept soaked with urine for months....as you can see, it's not exactly an elegant, simple, or clean process. Google some more on nitre beds if you're interested.
-
You may need a few weeks to accomplish the reaction in aqueous ethanol. It can even be done without the alcohol, but takes longer still. The main point of the ethylene glycol is that you can heat it strongly, which speeds up the reaction.
-
Is this decomposing PETE plastic? It'll work better with some water in the mix and ethylene glycol or diethylene glycol solvent, trust me. If you fuse with lye, it'll make benzene instead.
-
No, any silica glass (including fused quartz) or silicate ceramic will be attacked by molten lye. Stainless steel vessels are good for molten lye, but not if oxidizers are to be included. I recommend a cast iron crucible in this case, as a protective layer of magnetite should be formed. Molten lye with nothing added is surprisingly tame, but you need some serious protection if you want to add anything that may cause splashes or spatters.
-

Is dihydrothioctic acid the same as α-lipoic acid?
UC replied to venominme's topic in Organic Chemistry
Dihydrothioctic acid (dihydrolipoic acid) is the reduced form of lipoic acid, which does not contain the 5-membered ring of the parent compound. The S-S bond has been reductively cleaved to two thiols (-SH). http://en.wikipedia.org/wiki/Lipoic_acid http://en.wikipedia.org/wiki/Dihydrolipoic_acid -
BaF2 is close to insoluble in everything. How do you expect it to color the flame if none of it will dissolve? Red from calcium? Orange from sodium? Huh? Calcium gives a nice orange in my experience and sodium is the ubiquitous yellow.. You'd be better off spraying the solution through a flame, that way ensuring that the coloring agent is around when the alcohol burns. In a still dish, you're burning the fumes and the only way the coloring agent gets into it is by spattering. Cardstock burns yellow and is going to wipe out any other spectral lines you might see. A fused bead of salt on the end of an iron wire held in an alcohol flame should give you better emission for the difficult ones. Boric acid in methanol is great, because it forms trimethyl borate in equilibrium, which is volatile and can really color the flame a nice minty green. Ethanol is significantly less impressive and tends to give a mixed color flame of orange, blue, and green with boric acid, according to woelen. A good way to show off the purple line of potassium is to mix a bit of potassium chlorate and sugar and light it. This isn't really an indoors demonstration though. You won't see pink from anthing. Try some lithium for a nice red though. Copper acetate in methanol or ethanol is moderate for giving blue greens, but it's somewhat hard to tell the diffference from the alcohol flame. Indium gives a gorgeous, oddly saturated blue color, but it's not an everyday compound and teasing the color out is fairly hard. I think it is visible when I was burning a small sample of the metal in a propane-air flame.
-
so, you're sure that it's either NaOH or KOH? I'm only asking because Trisodium phosphate and Sodium carbonate are both frequently used for prepping and degreasing surfaces prior to painting. However, neither of the alternatives become rapidly "wet" from absorbed mositure when exposed to the air. My initial guess would be that you have NaOH since it's cheaper and just as effective for that kind of use. The problem with using a flame test is that the potassium emission line is very readily obscured by even traces of sodium. it's still worth a shot, because you can't get a false positive for potassium. If the flame is violet, it's definitely KOH. if yellow, it may or may not be NaOH, which brings me to this test... One of the quickest ways to get a definitive answer is going to be with a moderately concentrated sodium perchlorate solution. Take your mystery hydroxide and make a moderately strong solution. Mix the two. if it turns to the consistency of yogurt with gelatinous precipitate, you've got KOH. If nothing happens, you've got NaOH. This occurs because potassium perchlorate is soluble at a rate of 15g/L at room temperature. Sodium perchlorate is soluble on the order of two kilograms per liter of water at room temperature. If sodium perchlorate is unavailable, but you can get an authentic sample of NaOH, combine about equal weights of the mystery hydroxide and NaOH, melt, and take note of the freezing temperature, or remelt and take note of the melting temperature. Compare this to a plain sample of NaOH. KOH and NaOH form a eutectic, which melts at a significantly lower temperature than either one alone. If the unknown is simply NaOH, not much, if any difference should be noted. You could run a sample in solution (perhaps add an organic acid to lower the pH) through an AA spectrometer and look for K and Na. That's rather less accessible though.
-
What he said. If acid worked, they'd have been doing it for years. The fossil and matrix are for the most part identical chemically so mechanical removal is the only way to not ruin what you're after.
-
No common objects are made of tin (or were ever really made of tin). Tin cans used to be tinned steel, but they've been replaced by stainless steel and plastic-lined containers. Others are just made of aluminum. The closest you can get is pewter, which has antimony and copper added for hardness. It's relatively cheap to just buy bulk tin metal. Some places now offer lead-free fishing weights made of tin, but they are very expensive for how much metal you get. For larger quantities, I recommend this place: Knock yourself out: http://www.rotometals.com/Tin-Ingot-s/27.htm It does readily stick to steel and a lot of other metals when molten and it won't come off once cooled. Graphite is pretty standard for nonstick when working with metals though. Tin has a very high boiling point, unlike zinc, where the fumes are a hazard. I'd buy a ceramic crucible and use a bunsen and wire gauze for melting the stuff.
-
You know plain old metal sparklers? The cheap ones? Or do they not sell them up there? Just tie one to the end of a meterstick or a broom handle or similar and touch the thermite with the lit end. So far, I've had no problems lighting it like this.
-
Yes, it's called gluconic acid.
-
You're not going to make much dry ice that way, especially since that is a very small amount of CO2 and a lot of it is wasted cooling down the remainder to freezing temps. As a demo, you're in the clear though. You're probably better off with a small sewn felt bag than a pillowcase.
-
The wikipedia article does a good job of explaining. If you need something clarified, feel free to ask. http://en.wikipedia.org/wiki/Supercritical_drying
-
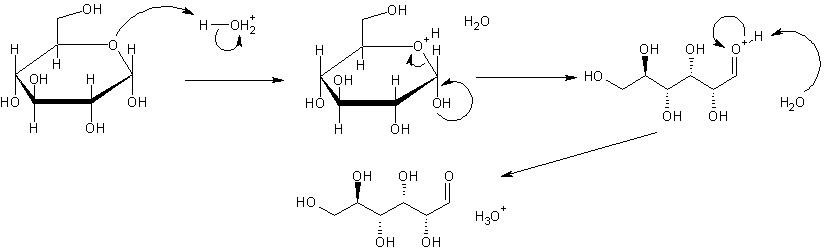
The pyranose (ring form) and chain form interconvert readily in water. Pay close attention to the attached image. I'm sure you can figure out the rest. On a technical note, those arrows should actually all be double-ended equilibrium arrows. The back-and-forth conversion allows for the interconversion of alpha and beta dextrose (which is what that molecule is). Reducing sugars are capable or ring opening to the chain form and have an aldehyde group. Do you see the ether-like portion of the ring and the hydroxide on the next carbon over? This hydroxide becomes the aldehyde group. The configuration is called a hemiacetal. You can look that up for more information. Nonreducing sugars have another ether-like bond formed with that hydroxy group (an acetal), preventing them from opening easily in this manner. As a side note, some ketoses (sugars with a ketone group in the chain form, instead of an aldehyde), which are intuitively not reducing sugars can interconvert into aldoses (ones with the aldehyde) via tautomerism. This is the case for fructose.
-
Go to a craft store and get clear (read the package! make sure the cured product is clear, many turn yellow) relatively quick setting epoxy, which might be referred to as jeweler's epoxy.
-
Use dry ice in acetone. It works quite well and is cheap. You may freeze out water from the azeotropic 95% by adding dry ice. Methanol is much better for flame tests as the flame is a consistent clear blue.
-
*bows* http://www3.interscience.wiley.com/journal/113374433/abstract?CRETRY=1&SRETRY=0 http://en.wikipedia.org/wiki/Helicene
-
That's because Rb and Cs are generally very expensive in comparison and don't really offer much bang for your buck. This page has videos of everything: http://theodoregray.com/periodictable/AlkaliBangs/index.html ^^^ yes, I realize he asked over a year ago, but just for reference. As for the sodium, take a look at these videos and tell me if you think this is safe to do with a group of students: http://www.periodictable.com/Stories/011.2/index.html The explosions scatter both unreacted sodium and lye everywhere, so plan on killing off a large patch of grass and building something to release the Na from a distance.