UC
Senior Members-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by UC
-
First point, I just learned something about silyl ether formation. Take back what I said about the TEA. It should work fine. It's all about stoichiometry. I however, get the feeling that for efficient protection of an alcohol, you probably need to use an excess of TMSCl, which won't give you what you want. If you use a strong base instead and make a monoalkoxide out of the vic-diol, you may be able to get a cleaner reaction. Then again, stoichiometry is usually not a great route to monoprotection or bromination, since you'll get some where there is no protection/bromination and some di-protected/brominated that need to be removed. A better route is by opening epoxides. Dry HX in an appropriate solvent should open epoxides to halohydrins. Use of alcoxide in dry alcohol (after addition of water or acid) will yield a mono-protected ether. Just as a note, vic-diols, epoxides, and halohydrins are somewhat unstable with respect to rearrangement depending on what the R groups attached to them are. Look up pinacol rearrangement and semipinacol rearrangement to know what to avoid.
-
Even if this is homework, the result of 100mg of ampicillin in 1ug of liquid sounds like wet ampicillin. Anyway, what you are looking to do is called dimensional analysis. For demonstration, I will convert 3 g/mL into kilograms per cubic decimeter (liter). To start off write your original units and note that it is a fraction with different units in the numerator and denominator. 3 grams / 1 mililiter Then, I multiply by a conversion factor. When you multiply fractions, the same units in opposite positions on their fractions cancel out. So, there are 1000 grams in a kilogram, so I write: 3 grams / 1 mililiter x 1 kilogram / 1000 grams = 3 kilograms / 1000 mililiters Notice that grams is in the numerator of the first term and in the denominator of the second? Once they cancel out, I'm left with 3 kilograms / 1000 mililiters. Now I convert mililiters into cubic decimeters, which I know to be liters. There are 1000 mililiters in a liter. To make mililiters cancel out, I put the mililiters in the numerator and the cubic decimeters in the denominator: 3 kilograms / 1000 mililiters x 1000 mililiters / 1 cubic decimeter = 3 kilograms / 1 cubic decimeter Not only have the mililiters cancelled out, but there was a 1000 on both the top and bottom that cancels out. The answer is written as 3 kg/dm^3
-

Org Chem - Lewis acids/bases and electron flow/curved arrows
UC replied to krock's topic in Homework Help
What is going to be the most acidic part of the ethanol molecule? The alkane-type hydrogens? The methyl carbon? The carbon with the hydroxy group on it? How about the oxygen? But it has those lone pairs on it....so what about the hydrogen attached to the oxygen? The O-H bond is pretty polar right? So, you should know what exact part of your ethanol molecule is the lewis acid (electrophile) now. The methyllithium is practically a lithium salt of a carbanion, so the carbon is going to have electrons it really doesn't want (lewis base, nucleophile). You draw arrows from the nucleophile to the electrophile, indicating that a bond is formed. The electrons in the O-H bond are going to migrate to the oxygen since the hydrogen is recieving enough to make a bond from the methyllithium carbon. So you need to draw an arrow from the O-H bond to the O, indicating that the bond is broken. The net effect is that the Lithium cation has moved to ethoxide and the methyl carbanion has gained a hydrogen forming it's conjugate acid.- 3 replies
-
-1
-
Yes, you have a mixture of KCl and KClO3. When heated, KClO3 decomposes to KCl and oxygen by the equation hermanntrude posted. The only thing that is going to leave the mixture is oxygen gas, so it means that 0.315g of oxygen is formed. What percent of the weight of the original mixture was KClO3?
-
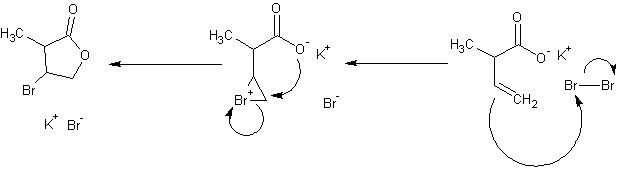
Did you mean cyclohexanol and any other compound up to 4 carbons? The monoprotection of only one aldehyde is not going to be a very clean reaction. You'll need a strong base though to make the enolate before adding the TMSCl. Triethylamine (TEA) is going to be way too weak. The organolithium is probably way overkill. A grignard reagent will probably be fine. Count your carbons. You have added an extra one after the organolithium treatment. The enol in the last step is going to rearrange into an aldehyde. I reccomend a wolff-kishner reduction to get to your final product. I have attached the shortest route I could come up with tonight. Contrary to popular belief, I am not a walking dictionary of organic reactions, so there are probably much shorter ways to do what I did. I am not sure if step 8 will jive with that halogen though, so it may need reworking. Reagents/reactions are as follows. 1: H2SO4, heat 2: N-bromosuccinimide, radical initiator (light, benzoyl peroxide, etc.) 3: dimethylamine, weak base (acid trap) 4: O3, Zn/CH3COOH (There are several other ways to cleave the bond to two aldehydes, but take more steps) 5: wolff-kishner reduction 6: Hofmann elimination 7: N-bromosuccinimide, radical initiator 8: Oxymercuration/demercuration 9: oxidation (wide choice of reagents) 10: Strong, non-nucleophilic base 11: Schlosser modified wittig reaction using 1-halopropane to make the wittig reagent Of course, it is absurdly complex to start from cyclohexanol. The second to last step can be reached directly with an aldol condensation between acetone enolate and propionaldehyde.
-
You could give activated charcoal a try. It's good at adsorbing organic molecules onto it's enormous surface area and tends to trap those in low concentration better than those in high concentration. Filter it through a cotton plug in a filter to remove the last traces of charcoal if you do manage to get it clear.
-
Yes, all those are the same compound that you list. Except Ive never seen it called scotch soda (sounds like something you'd order at a bar). I have no idea why they list all those sodium compounds in a profile on carbonic acid (basically CO2 in water). This ambiguity and vast number of names is why chemists prefer Na2CO3. Anyone who knows their chem will know exactly what it is immediately. Perhaps they won't know half the common names though, and confusion could arise if it was reffered to by one.
-
Most invisible inks are not Phosphorescent, but a few may be. The ink you used seems to be fluorescent, which is similar, but without the slow release of photons. Generally, glow in the dark items make use of a powdered phosphor either trapped in plastic or in paint applied to the item. Were there patterns on his shirt that could have been glow in the dark paint?
-
It won't need any catalyst at all This is done on a massive industrial scale with intact fatty acid triglycerides (veggie oil) to make brominated vegetable oil. The bromine addition makes the oil more dense as more is added, and they stop when it is exactly the same density as the drink it's being put into. That way, the tiny droplets of oil don't float or sink and the drink is "cloudy" looking which is desirable in some cases. If you can get any bromine, you can do this yourself by adding a drop or two to a little veggie oil and swirling it. The brown-red color vanishes as the Br2 adds across the double bonds in the oil. Eventually, you'll run out of double bonds though.
-
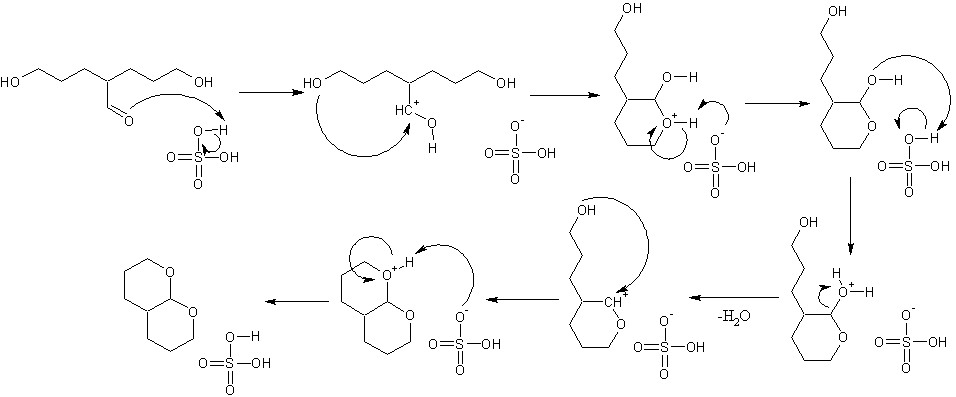
Even if you use just one equivalent of NaNH2, the deprotonatuion won't be clean. You will have a mix of mono and disubstituted acetylenes along with unreacted acetylene. Maybe we didn't cover whatever the sodium hydride was used for in class, and I haven't seen it elsewhere. Br2 in H2O is pretty bad at making bromohydrins, jsut because the bromide is right there when the bromonium ion forms. A better route is N-bromosuccinimide in 50:50 dimethyl sulfoxide/ water. After the bromohydrin formation, you use TMSCl. I assume this is to make a TMS-ether out of the alcohol which is on the second carbon from the end. NaOH is to eliminate the Br from the terminal carbon and make a TMS-trapped enol ether. You will get SN2 substution of the Br by OH before you get elimination. I would reccomend a very powerful non-nucleophilic base instead. Then you have anti-markovnikov hydroboration to give a primary alcohol. At this point, I'm not so sure...I suspect you are using the NaH to close the ring, cleaving the silyl ether and using PBr3 to make the free OH into Br. That last bit is really unnecessarily roundabout. Heating with a moderate strength acid will drive the ring formation since 5 and 6 membered rings are very stable. __________________________________________________________________________________________________________ Alright, had a look at your last PDF, and it looks very nice! You may want to substitute the Na in NH3 for a semi-poisoned hydrogenation catalyst and hydrogen. The classic one is called Lindlar's catalyst and I believe Pd on BaSO4 is also acceptable. A birch reduction (an alkali metal in liquid ammonia) is rather less pleasant that catalytic hydrogenation and both furnish the same product here. http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0606 You should probably use this instead of acetylene so you make sure you only get the monosubstituted product. I think K2CO3 in methanol will remove it and restore your terminal alkyne. That 1-bromoethanol you start off with is going to spontaneously eliminate HBr and form acetaldehyde, but if you can make the TMS protected form by some roundabout way, it will probably work. Potassium hydride seems overkill to make the potassium carboxylate. KOH or K2CO3 should be sufficient. And thinking through stuff like this is practically a hobby, so don't apologize.
-
Were you supposed to start with acetylene? I see issues with just about all of this, unfortunately. Acetylene is not a good candidate for NaNH2/alkyl halide treatment, because you'll get additions to both ends of it. The dibromide, after the first addition, is a secondary alkyl halide, for which acetylide anions act as bases to drive dehydrohalogenation, instead of substitution. For the second step, sodium will steal that bromine off of it's carbon, probably even more easily than it will reduce the triple bond. You'll end up with dimers coupled at that carbon, less two equivalents of NaBr. The third step looks fine, but you didn't write in the elimination of EtOH. For the last step, I don't understand why there is sodium hydride in there, nor why you are doing an anti-markovnikov hydroboration. I have attached what I'm thinking was meant by opening the bromonium ion.
-
Neither. You will get addition of both halves of the Br2 across the double bond, which makes a vicinal-dibromide (it is an anti addition if you are working with a ring system). If you want to pluck the COOH group off, you want to look up the Hunsdiecker reaction, which requires silver carboxylate salts to work. If the C=C group is around, you'll need to convert it into a nonreactive group that can be returned to a C=C bond later unless you won't mind both reactions happening simultaneously. By C=C bond broken, Im not sure what you mean. Usually this means, total scission of the carbon carbon bonds when I've heard it, but it's possible that you meant an addition of bromine across the double bond.
-
Yes, I use ACD Labs chemsketch, which I'm rather fond of at this point. I just need to convert files I save as .bmp into .jpg with another program, lest they eat up all my attachment storage space on this forum. Again, no problem. Did you get the one on cyclic esters? (called lactones).
-
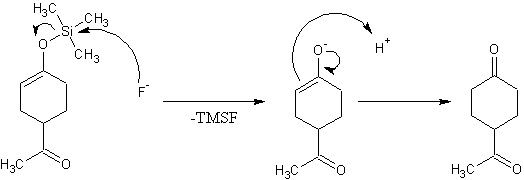
No problem. Things click for me when I draw them, and I rather like playing around with the drawings. Even wikipedia gives a reasonable explanation of tautomerism. To make a TMS ether, you need a powerful base to drive the formation of an enolate. Say, an organolithium, which is effectively a carbanion. The conjugate acid of the carbanion (alkane) is formed and the enol is deprotonated to a lithium enolate. Then TMS chloride is added, and the reaction proceeds like any other example of the williamson ether synthesis. LiCl is the side product. Somethign like "TMS ether removal mechanism" would have been fine. Chances are people will see it anyway and if they can help, will post.
-
After the TMS group is clipped off, you are left with a non-trapped enolate, which tautomerizes and snatches up a H+ to make the more stable keto form. It might happen how I drew it, or the enolate may first convert to an enol, then tautomerize. (This way was less drawing )
-
Open the ring up and restore the bromonium ion to where you think it should be. You should see a potentially nucleophilic group. You can make it more nucleophilic by deprotonating it. Even if there is water around, this group is right on the molecule and will preferentially attack the bromonium ion...of course it might hydrolyze afterward...so maybe you should consider a different solvent. If you still don't know, draw me your opened ring and Ill see if I can point it out better. PS: the attack is not on the carbon you expect. If it was on the carbon you'd expect from a typical intermolecular reaction of this type, you'd have a 4 sided ring....is a 4 sided ring a very happy structure? I'd think that the induced ring strain is probably bad enough to favor the non-markovnikov product in this case. A completely different way to do this would be to diazotize a terminal amine on the ring-opened structure. The attack on the resultant primary carbocation by the carboxylic acid is extremely rapid and results in ring closing. For rings up to 6 or 7 units, it tends to occur before rearrangement of the carbocation. I could dig up refs on this one if you really need. I'd tend to avoid it, since it's probably not taught in class and some profs may not even have heard of this, but it does have synthetic utility.
-
If you have a pile of NaHCO3 and add just enough HCl to theoretically react with all of it, but leave out a little bit, will you still have NaHCO3 left over in the reaction mix? What happens if you add more HCl? Im guessing this was a way to analyze your product. Hint: If you boil HCl, does it leave behind any solid?
-
Diliute solutions of silver nitrate (which are colorless) leave impressive purple stains wherever they touch skin, but I can't really recommend smearing them on yourself. Henna (lawsone) and the juice from black walnut fruits (juglone) are nice skin-reactive orangey-brown dyes that aren't toxic if you want temporary coloring.
-
* UnintentionalChaos gets a bucket and short stool * ydoaPs wonders why UnintentionalChaos has a bucket and short poo * UnintentionalChaos suggests ydoaPs reread that <ydoaPs> o.O You are missing half of the good stuff mooey...
-
Hrm. I don't expect this guy is coming back to check.... Here's the answer if anyone was interested. I left out the tautomers of the carbocation intermediates for simplicity.
-
I scored particularly average with a 20...my mental quirks are restained within chemistry though and when Im working on something. I can't (literally can't...Ill stare at the ceiling for an hour) and have no desire to sleep while I hunt references or am working on an idea....
-
Vinegar isn't that pure anyway. Your product will be discolored from organic crap that was in the vinegar. Distilled vinegar means the alcohol used to make it was distilled, but not the vinegar itself after being full of acetobacter and nutrients for the bacteria. The best way is to use a slight excess of vinegar and then boil it down, evaporating the excess acetic acid. Note that this will stink, (you know what I mean if you've ever heated vinegar before) but you won't have any carbonate or bicarbonate hanging around.
-
I think the problem is largely with progression and recording of ideas, as well as swansont's point that survival doesn't leave much time for science or art or anything. Being a genius really doesn't mean anything if you have no background knowledge...also you'd have to worry about being burned as a heretic for quite a while in history
-

Alien Plant Life and Sustainable Atmosphere
UC replied to BaronDestructo's topic in Evolution, Morphology and Exobiology
That's quite impressive about the fungi. So, it seems there isn't much of an upper limit on usable electromagnetic radiation, but I have serious doubts about the usability of lower energy stuff like radio waves. You need a certain energy input to be able to usefully excite electrons and radio waves are extremely low energy. -
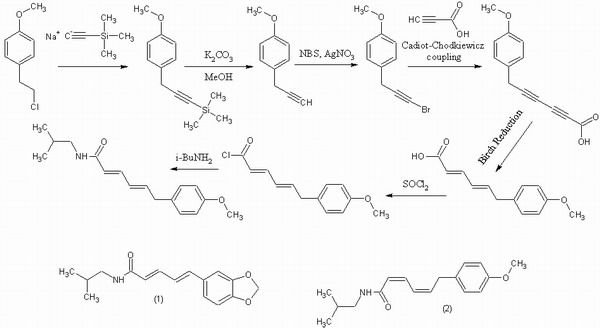
I'm not sure where to dig up someone who can make it for you, but I have attached a reasonable synthesis strategy. Compounds (1) and (2) at the bottom may also be of interest. (1) is a semisynthetic derivative of commercially available piperine, substituting in isobutylamine for the piperidine that normally constitutes the amide. (2) is the (2Z,4Z) isomer of your target compound, and easily produced by use of lindlar's catalyst for catalytic hydrogenation instead of a birch reduction.