UC
Senior Members-
Posts
547 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by UC
-
You already asked this on sciencemadness.org and had it answered. SOCl2 is thionyl chloride.
-

HELP ME need info on nitrates 4 sci. fair project
UC replied to Bananagirl6495's topic in Homework Help
Hermann has it right. They only affect the temperature when they are dissolving. Ammonium nitrate, for example, gets icy cold when you dissolve it in water. In fact, along with urea, it is used to make instant ice packs. Just make sure you let all the solutions come to room temperature before you start the tests. -
I'm not sure if you'd consider any of those things to be "complexes" per-se, which usually refers to transition metals with bound ligands. Perhaps the right word for what you want is a database of crystal structures? Maybe someone in the geology department (if you have one) could help if the chem. department is useless.
-

After shaving, does hair really grow back thicker and darker?
UC replied to cameron marical's topic in Biology
http://www.snopes.com/oldwives/hairgrow.asp Please use google once in a while. *facepalm* -

The Dangers of Staphylococcus Epidermidis
UC replied to Green Xenon's topic in Microbiology and Immunology
S. Epidermidis is clearly an extremely dangerous microorganism since it can live in environments containing the most horrible toxins. The key example of this is that it positively thrives in areas with high concentrations of dihydrogen monoxide, which we all know is a leading cause of death in humans. -
NaOH is a decent dessicant itself. Perhaps a very concentrated solution or paste of NaOH (excruciatingly hard to dry out, so the Ba(OH)2 will dry much faster) would be better to absorb CO2 rapidly and a tray of conc. sulfuric acid to dry the air. Using acetone is asking for condensation products when applied directly to a strong base. I'd shake with a tiny bit of ice cold distilled water so you lose only a small bit of barium hydroxide to saturate the solution and then suction filter through a frit and get it into that dessicator ASAP. If you want to build a setup to dessicate in nonreactive gas, I suggest you invest in a cylinder of nitrogen or argon.
-
You're not going to be able to determine much useful about that reaction at all. First of all, it's not a stoichiometric composition. There is an excess of nitrate there which could form numerous products as a result. Secondly, pyrotechnics tend to have very hard to predict products. That reaction will make sulfur dioxide and potassium oxide as some of it's products. These *might* form potassium sulfite, but they may also not due to the high temperature and dispersion of the mix. The dispersion of the mix will probably also prevent the reactions from finishing. 30% of it may go flying. It depends how the mixture is contained when ignited and the particle size of the reactants. The simplest stoichiometric mix with 3:2 ratio of sulfur to aluminum would be: [ce] 10Al + 15S + 18KNO3 -> 5Al2O3 + 15SO2 + 9K2O + 9N2 [/ce] or it may be [ce] 10Al + 15S + 18KNO3 -> 5Al2O3 + 6SO2 + 9K2SO3 + 9N2 [/ce] or [ce] 10Al + 15S + 18KNO3 -> 5Al2O3 + 3K2SO3 + 6K2S2O5 + 9N2 [/ce] Or some combination of these. There are also things I have not considered, like the possible formation of SO3 or sulfates. Even then, the reaction is not balanced.
-

Potassium manganate --> Potassium permanganate HELP
UC replied to jerryshizzle123's topic in Inorganic Chemistry
Add a weak acid like vinegar to bring the pH down and it will disproportionate to manganese dioxide and permanganate. Isolating it will be a pain in the arse. If you're a bit suicidal, you can bubble chlorine through the solution, but I seriously do not recommend that. Seriously. [ce] 4H^+ + 3MnO4^2^- -> 2MnO4^- + MnO2 (s) +2H2O [/ce] [ce] 2MnO4^2^- +Cl2 -> 2MnO4^- + 2Cl^- [/ce] -
No, but it's the acid anhydride of a weak acid. Points for picking at poor language choice, but it's not what was meant.
-
And I don't think you even can wave a chemical at it. if something can bind CO2 through simple reaction, it's usually made by kicking CO2 out of the compound (CaO by roasting CaCO3, for example) or has a hazardous and more dangerous sideproduct that needs to go somewhere. (electrolysis of NaCl in a cell with a diaphragm to make NaOH, for example yields chlorine gas side product.) And if we try to use petroleum to make any of the energy needed for those processes, we fall even further behind.
-
Whhhhhhattttt?!?!?!?!?! Also, methinks you're trying to explain this bass ackwards. The fact that the water molecules are ordered *is* lowered local entropy. It's not cause/effect. This is like saying that a lower temperature causes less vibration in the molecules. This is nonsense, since the vibration is the temperature. That heat had to go somewhere, so entropy overall will always increase (or stay the same).
-
AFAIK, it's not a true "explosive" so much as it vigorously and violently decomposes when catalyzed. If you were to put 90% H2O2 in an erlenmeyer containing even traces of copper, silver, manganese, etc. ions, you would readily make a rocket engine of several hundred degree steam and oxygen gas. chances are the erlenmeyer would shatter and scalding steam, peroxide, and glass would go be ejected in all directions, such as into your face. I don't believe you're working in a school lab at all. I think this is BS to cover your arse. High concentration H2O2 is made by reduced-pressure fractional distillation in dedicated glass apparatus, IIRC.
-
1) managed to reset my alarm clock, but a fire alarm at precisely the minute it was supposed to be set to woke me up and kept me from missing an important exam. 2) assurance that I won't do too badly in my electronics course, even though I keep doing abominably on the tests. Yay professor's discretion. 3) short extension on my chemistry research-related reports, so my workload for today is physically possible. 4) 1 day of classes left, with no real work in either of them. 5) accepted for a research position this summer at a local college so I can live at home and spend time with my girlfriend. The other option was to be stuck doing research at school for 10 of 16 weeks during the summer working 9-5, 5 days a week and driving 4 hours home every friday night and 4 hours back every sunday night just to see her for a little while.
-
Well, temperature is the macroscopic realization of the kinetic energy of molecules. I'm fairly sure that the answer you want involves the sharp and well-defined point where semi-free motion of the water molecules (water) is a lower energy state than vibrating rigidly in position (ice), but I don't know enough about this topic to say much. Ask a physicial chemist.
-
That's like saying "I'm going to eat this full course dinner so I have enough energy to run several miles to a McDonalds to buy a hamburger." It would be vastly more efficient to just use an electric motor, not to mention lighter in weight and far less complex.
-
Carboxylic acids are reduced to the alcohols as well, but one equivalent of hydride will be wasted deprotonating the free acid. For example, http://www.orgsyn.org/orgsyn/prep.asp?prep=cv8p0434
-
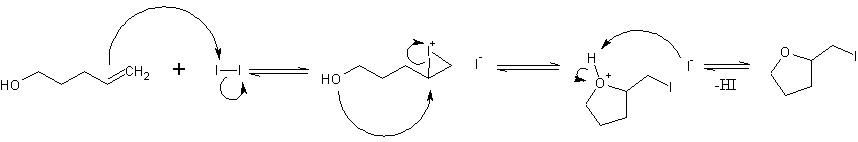
Iodine is very poor at adding to double bonds, so perhaps the first two stages normally equilibriate, but don't proceed to full addition across the double bond. I'm just guessing at that, but that's probably why iodine is used instead of other halogens, which would readily add completely across the bond forming vicinal dihalides. Iodolactonization is also known, where the alcohol group is replaced by a carboxylic acid. The iodine sources I'm seeing are N-iodosuccinimide, I2, or a mix of NaI and FeCl3 with solvents like acetonitrile and chloroform at 0C I don't know how well this reaction would work intermolecularly, but my guess would be poorly.
-
No, technically HCl (g) is not hydrochloric acid. Hydrochloric acid refers to HCl (aq), although the term is often stretched to cover solutions of HCl in other solvents. Ksp is for ionic solids. There is a solubility constant for non-ionic solids as well, but it's not Ksp. Theophrastus- please check the date on a post before responding to it. The last post was 3 years and a month ago. I seriously doubt that Derrell is still doing this homework. For anyone who cares anyway, as was suggested by the other posters, look at what kind of substances HCl, NH3, and H2O are. Like dissolves like. You may also want to search for "MSDS HCl gas" or similar and check the water solubility under the physical data section, to give yourself an actual number.
-
No. Please learn what this means and how to use it: http://openlearn.open.ac.uk/file.php/3258/T357_1_ie001i.jpg "Galvanic series" or "electromotive series" are helpful things you might punch into google, which I will not do for you. Granted, everything is an equilibrium, so transiently and randomly, some magnesium might make a few sodium ions into sodium metal, but they will immediately give up those electrons to water or back to the magnesium ions that were formed.
-

Are there element in the surface of the Sun which do not exist on Earth?
UC replied to elektrisk's topic in Homework Help
It's not so much that it's so unreactive, as argon was discovered in the atmosphere, but the fact that there isn't much in the way of sources for it. As far as I know, all of our helium is from natural gas sources. The helium is the result of alpha-decay of thorium and uranium minerals (the alpha particles slow down, capture some electrons and become [ce] ^4He [/ce]). It becomes trapped in the same impermeable pockets of rock as the natural gas. I believe a uranium mineral that had the gas trapped in it directly was responsible for the original discovery on earth though. -
3% H2O2 and slightly rusted (the rust seems to be catalytic) iron turnings, IIRC will get hot enough to boil/produce steam for a good long time 15% H2O2 with activated charcoal "bubbles" a whole lot (oxygen) and probably will eventually get hot enough to boil the water involved. The decomposition is fairly slow when I used the big chunks of carbon meant for fish tank filters. If you mean "how can I make what looks like a plain glass of water boil for a long time," I have no idea if I can help you. You could aways pull a vacuum on it and it will really boil as long as you keep pumping out the water vapor
-
Yes, this is a simple metathesis/precipitation reaction. Keep in mind that barium compounds are quite toxic and the hydroxide is very sensitive to reaction with [ce] CO2 [/ce].
-
It's the solubility product equilibrium constant. It sounds to me like sjlopez is in analytical chemistry or at least a course that touches on some analytical concepts. Can you please explain the experiment better. The calculations are probably trivial, but I'm not sure what you did with NaOH or the HCl.