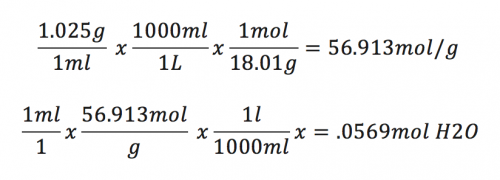-
Posts
51 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by FrankP
-
I understand what you are saying. I'm not dumb, I get it, I have 3 weeks left in the semester. The point of me picking up physics books and re-learning everything would make no sense. As it is I am still in the top 5% of this class in terms of grades. This class is an introduction to chemistry. For undergraduate students at a non-technical institution where less than 1% of all majors granted on a yearly basis are in the field of science. For this teacher to assume that any of the students she is teaching know what you are insisting that the average person knows is a fallacy on your end. Please either contribute to the topic or back out of the thread.
-
Thanks for that tidbit of your personal opinion there. Contributions to the conversation in a positive direction seem to be your specialty. If you do not plan to assist in the conversation then I will sincerely ask for your resignation in this board. I lack general knowledge because it has been 10 years since I have taken a class which required this knowledge. Also FYI this is a chemistry class. Being that this event is occurring under water I understand that g=gravity and I know 9.8m/s^2 is the value for gravity It would not be my first guess. Had this been a balloon accelerating from a kids hand filled with He gas I would have considered Gravity in the equation so pardon my inability to draw conclusions being that we have not even mentioned gravity one time in my chemistry class 'it must have slipped my mind'... ok so I tried this but the problem is I don't understand how I am supposed to integrate gravity. We have never done anything like this.. I have looked on every website and everywhere in my textbook and I have not seen anything that would consider this question chemistry.... So I will try and solve it using the formula you provided me.. Boyles law says volume and pressure are inversely related. How to adopt that to 100' depth of seawater I am not sure. I don't understand how to mix the two together, though. How can I multiply (1.025g/cm3)*(9.8m/s^2)*(100ft) all those units are inconsistent. If I convert 9.8m/s^2 to Centimeters its still going to be squared not cubed. and if I convert 100ft to centimeters in height I will have the following units ( Grams, cm3, cm2, cm) i understand when you multiply you can add exponents but does that really apply to this?
-
No I have never seen that so its P= (1.025/1cm^3) x (grams) x (100') Where Pressure and Grams are unknown the only other way I can think to get grams is this: (Density CF) * (1ml -> L CF) * (MW H2O CF) = (Moles H2O / L) (Moles H2O / L) *(1liter/ML) * (1ml/1cm3) = (.0569 mol H2O/ Cm3) (.0569 mol H2O/ Cm3) * 2.832x106 = 161140.8g H2O Total in the entire 100 ft3 column. To be honest the math I just did above to me sounds horribly wrong it sounds like im just trying to do something to get a value for grams lol that is what is getting me pissed. Im literally playing a guessing game now... the file I attached below has already been attached but I took the number of 56.913 mol/liter (that is a type mole/g sorry about that) that I got and took it one step further to get (g H2O / Cm3 so I could use that conversion factor to multiply by the Cubic volume of the entire 100 foot column. How about this I tried to use. Your idea to find the total pressure at the bottom I'm not sure if this is right
-
Well we just touched on Boyles law in passing today we hadn't actually done any problems with it so I guess that might help me let me check it out and get back to you... Boyles law assumes that 2 of the variables remain constants right? Assuming an inverse relationship to volume and pressure.. Oh man... lol lemme see if this works ___________________________________________________________________ Ok so I get what you said here P1V1=K1=P2V2 or more ideally --> (P1V1=P2V2) I get this equation but my question is I don't know the pressure or the volume but now im stuck just with a different equation I am able to get moles of H2 O and moles of Mercury using the density and that is the only mathematical calculations I can perform without using google to tell me the pressure at a depth of 100 feet. Which I do not want to do because I know that I won't be able to use google on my exam.
-
This is an interesting spin on how to think about this problem are you suggesting you set one of the variables such as T = 1 since it is not given in order to help solve for P? I understand what you are saying about comparing arbitrary V1 to V2 I get the concept but to me that is not accurate. I am prob wrong but when you don't know the displacement of the bubble you can not determine accurately how much larger it is. It is at best an educated guess. But that's not my main source of confusion. To answer your questions What is the pressure below sea level I don't know. I believe you are asking me what I asked back again. I don't know what it is BECAUSE I don't know how to calculate it I know the pressure at sea level (1 atm) Pressure at 100' depth and pressure at sea level are connected because the amount of pressure being exerted on the outside is less as depth decreases, therefore the volume of the bubble will increase. As the bubble approaches the surface. Let me re-explain my confusion because I kind of feel like this happens a lot here, I ask a question explicitly state where my confusion is. Instead of being shown how to approach the problem I am asked the same question that I asked, with different words. I don't understand (PV=nRT) enough to deduce this kind of information I just learned it on Wednesday for the first time (Incase there is time zone differences I posted this question Wednesday, there is no way this is considered beginner problems). I am not given temperature and I am not given pressure at the 100' depth. Therefore there are 2 unknowns in the equation. It mathematically is impossible to solve with 2 unknowns (unless an arbitrary number is substituted for the one variable). In my example which is attached, I tried to substitute (T=1) basically making the equation PV=nR [or PV=nR(1) ] to try and solve for pressure at depth. This did not spit an answer back that made any sense, however looking back maybe that's all I am being asked to do. (But I highly doubt it with my teacher)
-
As usual, I am having a real tough time with this... I have attached my work I have done and the questions. PV=nRT where R is a constant @ ((.08026 L) x (atm)/ (K) x (mol) ) So if I could air out my thought process here to so you guys could help me determine what I am doing wrong in my thinking. I am looking at the column of air in which the bubble passes as a prism so I can gather the volume. I know that the density of both H20 and Hg are given and are 1.025g/cm3 & 13.6g/cm3 respectively. So now that I have density I can gather some information in terms of D=(Mass/Volume) Therefore: H20 ((1.025g)/1cm3) or ((1.025g)/1mL) ((13.6g)/1cm3) or ((13.6g)/1mL) Using that to further explain the column of space that the bubble travels you want to find total moles of H20 present in that space. So finding the overall volume of space is necessary. I am not sure about Volume this is the 2 things I have tried: I looked to find V in terms of 100 ft3 because it is unreasonable to assume that the space which the bubble passes through is 2D (at least that's how my brain thinks). 100 ft3 = 2.832 x 106 cm3 and using that information you can gather total moles of H20 contained in that area. Use the density as a conversion factor as stated above. Where: D(H20) => ((1.025g)/1cm3) or ((1.025g)/1mL). If you take that factor and multiply it to get moles per liter in doing so take the conversion factor and plug it in like this (image attached #2) it's a conversion ratio between grams of H2O in the conversion => Liters => Divide by molecular weight of water. This should get you # of moles per liter. Then you need to find out how many moles are present without the liters. So you end up with .0569mol H2O OK... so now I am done with my ability to rationalize the answer. The reason I find myself so confused is that we are not given a size of the bubble or temperature. I want to be able to say that temperature should be consistent even though I know there would most likely be negligible temperature difference in terms of this. I know the temperature has to be greater than freezing. However, I'm not sure if there is a specific temp that is assumed for problems like this in the value of water. If that is the case I am making this harder... The second point of confusion is the relevance to Hg Mercury as ((13.6g)/1cm3) I know that barometers are usually measured in units of mercury as a standard and so I know that I need to use that to eventually help me solve the problem but how? Thanks as usual
-
I have come to this realization. I am still determined to master my course but it is going to be quite the struggle. Thanks for the help again.
-
Ok yes sorry, this can be extremely unclear. I was able to figure this out using the suggestions. I have gone to my teacher's office hours as I usually do but rather than ask lecture based questions I decided to dedicate time to the lab. If you don't mind I will post my findings and let me know how I can become more efficient at obtaining this kind of information. What I had meant was we added 50mL of .1M NaOH solution. We added approx. 450mL of water to dilute it. From there we performed a rough titration and 3 trial runs to standardize the concentration of the new 'diluted' solution of NaOH. From this, we performed 3 basic trial runs, bottled up the NaOH Substance with our names on it and stored it until today when we used that standardized substance to perform titrations on Vinegar & Anti-Acid tablets. So the problem was from what I can understand was that the formula for the chemical reaction was not given. Since I am not good at creating chemical formulas out of thin air, (because we have not gotten to that part of my course yet) I was unable to identify that the mole ratio was 1:1. I have also attached my sheet of work, where I (to the best of my abilities) worked out the solutions to my questions. I wanted to attach this work file as a way to redeem myself for asking such rudimentary questions. [i know most if not all members of this forum are much more advanced on these topics. I feel a strong desire to get better at chemistry. Chemistry and physics are 2 of my favorite subjects I just lack formal education in it as it has been 10 years since my last chemistry class. So for that, I apologize I do want to advance my skill in the desired feild]
-
I agree, however, I am not quite sure how your response is supposed to help me with my question. Suggesting I pay close attention is not why I asked the question. I'm a 25-year-old undergraduate student I am paying close attention trust me. My question is specific I could use help with it, not suggestions on how to achieve academic success. I appreciate your input but would appreciate it more if I could get appropriate responses.
-
This lab makes no sense to me. The information I am given and the information I am being asked are to abstract for me to figure out this early in my understanding of these concepts. Problem is Lab and Lecture are taught by different teachers at my school. My lab professor follows the book Lab 1-9 in order. Problem is my Chem Lecture professor does not. She teaches how she wants. We just learned molarity this week. We just started it no learned that's the wrong way to say it. I barely understand it and now I'm being asked to calculate these answers and I have no idea how to do it. I'm going to scan this and attach it. Here is whaIs known that may not be apparent from the pictures I added. NaOH we used 50mL of .1 M solution Diluted it to 500mL of NaOH solution with an unknown concentration I can't figure out how to find moles of NaOH My work so far: I did (V1)(C1)=(V2)(C2) (50mL)(.1M)=(500mL)(C2) I got .01M NaOH I know this calculation is right however the problem is in order to calculate the moles of NaOH I would have to know that the 50mL was exact which I can not prove that I got it as close to perfect as possible but i'm willing to bet it was at least .01mL off. Which the purpose of this lab is to figure out the values as specifically as possible so I don't want to guess... My other question is that I set up a problem
-
How's this? Did I do my work right? It won't let me attach the screenshot of my work I scanned the paper I worked the problem out on how do you attach it?
-
Ok so I'm following what you are saying. However, I'm still stuck where do you start. This is the first day im doing these kinds of problems so idk if its wrong of me to assume that this problem is too hard for me but im going to assume that anyway. I know you can get conversion factors from the problem and all that but how am I supposed to get anything out of this problem. Nothing is given it's saying hydrocarbon solution which means nothing. Everything is a hydrocarbon solution. To me, this problem says this. "You are at a car dealership with $200 in your pocket. There are 2000 cars at the dealership. 300 are red. You're father was born in before you were. What state do you live in?" None of the prior information builds off of anything that comes before it, well at least to me. I know you guys are trying to help me and I'm following all of your logic. My problem isn't that im not smart enough to figure out what it's asking me my problem is again... I don't know how to set up the solution to the problem because I just learned this on Wednesday. I'm 25 years old and the last time I had chemistry was 2006/2007. To me, the problem is this... The chemical reaction showing in the formula shows me nothing because its ionic compounds mixing together which I understand that the formula is showing you what was meant by this statement "Reactions were carried out on a 2.017 g sample of the solution, which converted all of the chlorine to chloride ion dissolved in water" but what the hell does this h ave to do with the C10H6Cl8? Just simply the fact that 8moles of Cl will be converted into "chloride ions?" I could, however, I can't get past the value 100 (X/2.017) what's the 100 representing why am I using that. I'm very skeptical when it comes to starting problems with arbitrary numbers. One last time. I don't know how to start the problem. Therefore what I have done so far is attempt to start the problem with no avail.
-
To be honest, at this point I have no clue I have tried it several times and I can't arrive at an answer at all I cant figure out how to set the problem up
-
We're just beginning stoichiometry and molarity we have been on this chapter for 1 week now and this is a homework question. I have thought about how to start this problem several times so far and I have decided that I have no clue. A chemist was asked to analyze a solution of chlordane, C10H6Cl8,dissolved in a hydrocarbon solvent that was discovered by construction workers during demolition of an old work shed. This insecticide was banned for sale in the United States in 1988 by the EPA because of its potential for causing cancer. Reactions were carried out on a 2.017 g sample of the solution, which converted all of the chlorine to chloride ion dissolved in water. This aqueous solution required 72.61 mL of 0.2020 M AgNO3 to precipitate all of the chloride ion as AgCl. What was the percentage of chlordane in the original solution? The precipitation reaction was Ag+(aq) + Cl-(aq) → AgCl(s) I know I can use the information here to get G of AGNO3 and also get a few other things but the problem is I don't know how that relates to the chemical equation provided. I keep thinking this is a limiting reactant question in the sense that they are telling you "This aqueous solution required 72.61 mL of 0.2020 M AgNO3 to precipitate all of the chloride ion as AgCl." The only thing I can think of that 72.61 mL of 0.2020 M AgNO3 is the limiting reactant and so using that I could transition to total potential product possible and use that conversion factor to try and solve with this second part of the question. Either that or like I said above I have no clue as I suspected. lol
-
Ok thank you for the help, I was wondering what key terms would yield the best results.
-
Yea it is really difficult for me to do that i'm glad I am not the only one who doesn't find this to be super simple! Anyhow I had a part 2 of my question one of my worksheets says this... How many sub-shells in the 7th main energy level? How many electrons in this shell? What is the difference between the Px and Py orbitals? My attempt to answer this: a) When N = 7 L = 0, 1, 2, 3, 4, 5, 6 (meaning 7 sub-shell's) 0=s, 1=p, 2=d, 3=f, 4=g, 5=h, 6=i b) max 26 electrons in i subshell Max e- allowed in 7th Main energy level (98 max e-) s=2 p=6 d=10 f=14 g=18 h=22 i=28 c) The difference between Px and Py orbitals is the way they occupy a 3D space. A 3D space is graphed using an X, Y & Z axis. When an orbital such as Px is graphed it occupies/aligns itself with the X axis. VS a Py orbital which would align itself with he Y axis of the 3D space/graph How am I doing? I found this question to be tough but as I reasoned through it just now I believe I am confident in the answer... Problem I have found on this is that my book and internet sources have never really explained what goes on past N=4 We have made mention of G orbitals but have never quantified a maximum number of e- for it but I tried to recognize a trend of +4 per sub shell and therefore guessed that the max for i sub shell would 28.
-
So in my lecture we blew past energy systems today and its been a while since I studied them and to be quite frank it was anatomy and physiology so the concepts of each energy system were as deep as we got. We studied net gain and stuff like that but never detail. I am just slightly confused in the aspect of the energy systems when H+ gradient is created and protein synthase helps in the production of ATP molecules though the electro-chemical gradient. I am wondering how does the cell pump H+ ions though using NADH. Is there any good videos or maybe someone on here could point me in the direction of a good place. Thanks
-
This makes sense you know what is actually helping me make this click. I watched a quick video lecture and in there they explained that the ML value is actually related to how to graph it mathematically.. Pz Py Px etc. That made it make more sense to me and so L is explaining that shape/type of orbital that the e- will have, while ML States how many ways that orbital can be "graphed" or tracked idk if graphed is the proper term. And then we use those -1,0,1 values to place electron spin within in order to calculate Ms which is either +1/2 when unpaired and -1/2 when unpaired. See my problem is memorizing things is hard for me so I like to try and make it make sense in a grander picture that helps me remember things when things make sense to me. I find I have the hardest time with memorization without application. I think I am getting it to finally click though however you guys will let me know if is of any truth haha
-
I have actually read a few studies that stated that short term memory can be improved. In a study people were read random sequences of numbers in no particular order and asked to restate what they had heard. After 6 weeks short term memory function was improved drastically. They also stated that over the 6 week period there was some indication that short term cognitive recollection improvement posed equally beneficial to long term memory retention I will look for the article if you would be interested in reading it... As far as games go aren't there websites claiming to improve brain function? like Lumosity or whatever its called?
-
I mean the best way to remember each of the 3 for me is this... Aufbau- Building up electrons to find ground state Hund- is that all electrons must be placed into sub-shells before they can be paired. Pauli Exclusion- no 2 electrons can have the same 4 QN Aufbau itself is an abstract word when compared to normal english vernacular this should be able to be memorized on its own Hund's rule you can think of the word hugs. there needs to be 2 people to hug. Therefore you can analogize that all electrons must be placed into a sub shell in order to hug another one... something like that Pauli Exclusion the word exclusion in this should throw up flags as to what it entails. Its exclusive each electron is exclusive and therefore no other electrons can have the same SSN as another one... Lol just trying to help I am not a pictorial studier or anything so this might help might not.
-
Were not given any more information then this unfortunately I don't know how I am supposed to solve it either. I follow your reasoning when solving the problem and it actually makes more sense but like you said I am not sure how we could solve that given the title info we got. So we determined in class that the values that I posted were for Z=30 The only way I can reasonably explain what my teacher has done here is to say that since she states mL = +2 Ms= -1/2... That would mean that +2 would place the last electron in the 5th sub shell and being that the spin is -1/2 that would mean that all electrons were paired in this 5th sub shell since this was the 5th sub shell in the d orbital we could assume that this element would have the maximum number of electrons in its outer shell, 5d10 and then we would have to find the element that has such a electron structure? does that make sense or am I wrong here too lol I think given your explanation it makes more sense now for me to follow along. If I could ask a stupid question when you said "and l = 2 (this describes the type of orbital; l = 0 for s, 1 for p and 2 for d)." how did you know this? I feel like I am struggling because ML and L never seem to make sense to me. My book says L is n-1 but thats never the case... and then ML I obviously have no clue how to get that because I can't even get L
-
Hello, So I am in general chemistry and I have had a long absence since my last chemistry class which was high school back in 2006 so I have forgotten general principles. My teacher has her PHD in it so to her this seems simple, my question is two fold. When using Aufbau Principle and mapping out the 1s2-2s2-2p6-3s2-3p6-4s2-3d10-4p6-5s2-Etc... Is there an easy way to determine the valence election shells? My book mentions in passing that the valence shells are determined by the group/ period that they are located in but it does not explain that. The homework I am fine with but I have a test coming up next week and so I am preparing in advance. I have to do a few problems where I find valence shells I can do this by writing out the whole Aufbau chart and then doing the math to add up to the total number of electrons. Just wanted to know if this is the only way. My second question is when you determine the atom you wish to map out so say for example my teacher does this (which I know she will because she told me she will lol) n=3 L=2 mL = +2 Ms= -1/2 We determined that Z=30 and therefore the element is Zn but I don't fully grasp how to reason my wa to getting that answer. Thanks in advance for the help! Frank
-
In my opinion i think that google+ is way before its time... Facebook is at too much of a high right now and the way the google+ is setup makes it too difficult for friends to link up. Its more of a Twitter meets Facebook and has a slow offspring. That would be google+. But I think that as the fan base grows then it will be better... but for now not so good!