Dan_Ny
Senior Members-
Posts
64 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Dan_Ny
-
Actually, this is a question that haunted me a long time. Until I read about Heisenberg and got somewhat disappoined. The major issue from a positivistic point of view might be: Is it really worth it to make a model which is nearly as exact as the original (for example your brain, which you want to predetermine)? Because it cannot be as complex as the brain itself. Why? Because the brain in not just the brain, it is influenced by it's environment, the universe. So, in order to build an exact model of the universe you would have to make something that is at least as big and complex as the universe itself. Ans here comes the positivist and says: I have a THEORY. A theory is an assumtion, a simplified model, which can make heuristical predictions that are - most often - correct. If the predictions are incorrect, the positivist throws the theory away and tries - based on empirics (simplified experiments on parts of the universe, approximations essentially) - to get a better one. His theory is: If I give this brain a specific impulse - fear - and it will, according to experience - make it's body run away. But he could be wrong, of course. To be sure he had to know everything - since everything is connected by the fundamental forces of physics. I guess.
-

number of layers in a surface of a substrate
Dan_Ny replied to sfpublic's topic in Inorganic Chemistry
Precisely. -
thank you cap'n. your words are a blessing for my coffee-drunken soul.
-
dude farmboy - too many words dude let me try it shorter so äh tropicalmango, when you wanna work with a naoh solution with a specific concentration, make sure it is completely dissolved. otherwise your stuff will not have the concentration you want, because there is some solid lying around and not bein in solution where it has to be. ah and take koh next time. it works the same but dissolves thousand times faster. and btw. wear lab glasses. please.
-
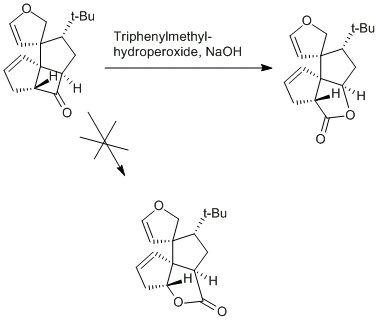
So guys I had a look at Corey's 1988 synthesis of Ginkgolide B and came over an innocent-looking step - a Baeyer-Villiger-reaction...: Now, it took me a while to see, why it proceeded totally regioselective. Who knows the answer? (I mean, besides me ) (Note: I is not going to be the "usual" reason of migration preference, since the carbons next to the carbonyl are both secondary alkyls...) Hint: From which side is the peroxide going to come from?
-

macrolactonization vs. ring-closing metathesis
Dan_Ny replied to Dan_Ny's topic in Organic Chemistry
Wow. I am beginning to like this stuff. Hm, so Grubbs also has it's disadvantages. And enzymes - I definitely don't know enough about biocatalysis. Are enzymes allowed to be used in total synthesis? Or ist it "too biological"? The problem might be that you don't know if a specific enzyme also works for your substrate.... By the way, do you know how they get regioselectivity for the sugars in erythromycin D? -
To get large rings (with a endo lactone functionality) closed, the traditional approach has been to use macrolactonization. Because the entropy factor overrides the enthalpy factor for large rings, suppression of the intermolecular reaction becomes more difficult. That problem was addressed by activation of the carboxylic or alcohol moiety, for example by the use of thioesters to "push" the reaction against entropy by increasing the enthalpy (correct my, if i am wrong here). in the last time, i came over an immense number of examples where rings were closed by ring-closing metathesis (thanks be to grubbs). is the time of macrolactinzation finally over? opinions, please!
-

Whats the most dangerous chemical you have used / seen?
Dan_Ny replied to RyanJ's topic in Applied Chemistry
Cruel. I know why I wear my lab glasses. -
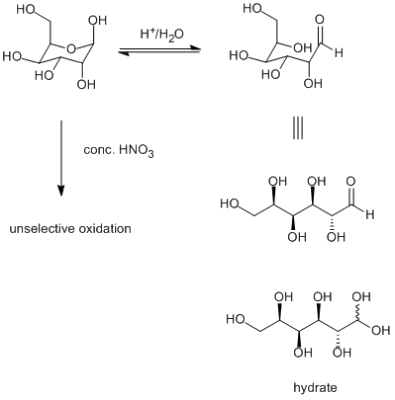
You are great, thanks a lot, horza2002! Actually, the re si thing is much easier than I thought ;-) I just looked up the third kind of chirality, planar chirality (because I finally wanted to get that in my head, too). According to wikipedia (http://en.wikipedia....lanar_chirality) wow. I could not have been putting this better myself tiny - there are two things that might happen if you treat your carbohydrate with HNO3 (also depending on the concentration of your nitric acid). At very low concentrations and as an aqueous solution, the sugar will (acid catalyzed) eliquibrate and exist partly in it's open chain form (and, to be precise, in the hydrate form of the aldehyde, if i'm correct). Now, look at the picture below. How many chiral carbons do you have in the open sugar form? Does the number differ from the closed form? The second possibility (which I like because it shows how rude and unfriendly these inorganic acids are ), is, of course, that it will rip your sugar apart. HNO3 is a strongly oxidizing acid and I don't know what it's gonna do, but you won't recognize you sugar after it. Or your stereocenters. Ah and like horza2002 said, in the closed form it's five, not four stereocenters.
-
Hm, that's interesting. What conditions does it take to open up a 6-membered lactone? (Or, to which conditions are 6-membered lactones in a substrate stable?)
-
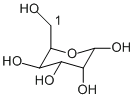
Well, I know that you know, Horza2002. Just giving you a hard time. Yeah I understand and you are rigt, knowing the conformation can make it easier to see the priorities on a ring carbon. Well I definitely forgot everything about axial and planar chirality but this would be the ideal time to look it up again. And what was that stuff with the re and si face of trigonal or prochiral molecules? Hm, I should know that... The structure was actually for tiny529, I don't know if it is any help, though.
-
Ring conformations have no influence at all on the configuration of the stereocenters, so, even if you have to assign the R/S notation, the conformation does not matter. Now, I don't know how your structures look like, but what horza said, four different substituents on a carbon, applies also for carbons in a chain or a ring (like in sugars). So, look at you structure - it should have one oxygen in the ring and a CH2OH group attached to the ring next to that oxygen next to it and looks perhaps a bit like this picture: Well, does carbon 1 have two substituents that are the same? So, ist it optically active? Now look at the carbons in the ring. How many different substituents do they (all) have? Careful, it is sometimes not enough just to look at the next atom. (And here is a bit more about the CIP rules and optical activity: http://tigger.uic.ed...xt/chapter5.htm )
-
I think that critics against coffe fell out a bit hard here. I've never observed any additcion with myself, even when I stopped drinking coffe for more than a week a couple of times - pherhaps i was a bit tired the first couple of days compared to the usual coffee-driven awakeness the days befor, but that's about it. I did not want to give the impression that it is my believe every chemist is a coffee drinker, horza, rather just wanted to point out the fact that coffee is used by many of my labmates and by myself as a tool for example to get awake some morning. My ideal picture of the responsible coffee drinker is, in the end, someone, who knows the benefits of this unique drink and is able to use that to his or her advantage (e.g. in times that require high performance). If you don't drink coffee regularly, but only, when you want to be productive, imho you can benefit most.
-

Palladium-catalyzed intramolecular cyclization
Dan_Ny replied to Dan_Ny's topic in Organic Chemistry
Even though that is logical, the reaction proceeds perfectly if toluene is used. -
It is quite common between chemists to consume large amounts of coffee while doing lab work and/or writing scientific essays and I just wanted to ask everybody how they feel about the application of high doses of caffeine in science. After all I heard, the whole issue is double-edged. Naturally, being one of the most healthy stimulants, caffeine will give you and your mind a burst of energy to perform well (especially in the morning). Plus, coffee is said to contain a number of antioxidants which have a positive effect on your health and metabolism. On the other hand, caffeine certainly draws water from you body which - unless compensated - never is a good thing. Now I am not sure about the long-term effects of caffeine to various organs of the body, first of all the brain. Does anybody know more about that? Just from my feeling, it can't be good. But once you're addicted... well. I would be happy to see the opinion of others regarding this highly important scientific issue!
-

Palladium-catalyzed intramolecular cyclization
Dan_Ny replied to Dan_Ny's topic in Organic Chemistry
Thank you for the answers, guys. Yeah, that sounds reasonable. Do you think that sodium would coordinate more to the enolate than potassium and thus being more soluble in toluene? I should try to find out if the reaction works faster with lithium, then... -

Whats the most dangerous chemical you have used / seen?
Dan_Ny replied to RyanJ's topic in Applied Chemistry
horrible tutors, yes. i remember my OC tutor from my third semester as an undergraduate. horrible is a friendly word for what he was. or is? -
Beare, N. A.; Hartwig, J. F., J. Org. Chem. 2002, 67, 541. Well, inspired by the methodology from the paper above, I tried to make a palladium-catalyzed intramolecular cyclization on a substrate like this: When I use KOtBu as the base, nearly half of the product is the hydrodehalogenated one (the one below). Cyclization occurs quantitative, though and without side-products, when I use NaOtBu. I totally do not understand that. The solvent is toluene. Na3PO4 does not work at all and K3PO4 works as good as KOtBu. Can anybody explain these strange results? Thanks, Dan
-
A [2+4] cycloaddition? Are you talking about my favourite reaction?
-
Hm, as far as scifinder goes, pachypodia natural products have not yet been isolated or identified. But correct me, if I am wrong. I found this here, which says the arrow poison might actually come from different plants, since they are a mixture of plant extracts, but its a very old article. http://onlinelibrary.wiley.com/doi/10.1002/hlca.19640470824/abstract Anybody else found something?
-

Whats the most dangerous chemical you have used / seen?
Dan_Ny replied to RyanJ's topic in Applied Chemistry
I remeber being an undergrad and having to use half a gram potassium cyanide for a benzoin condensation. :D Awesome... -
Yeah, I imagine that chiral solvents, which must be damn expensive (and, like you said, useless), must be even more expensive (and even more, like you said, useless) when deuterated. A shame, the idea using the solvent as the chiral environment neccessary to distinguish or generate stereocenters itself sounds interesting. -- Sounds also pretty damn convincing Here I take the liberty to extend the "chiral adjuvant/auxiliaries" you mentioned by the substrate itself (once we have that enantiopure). Before the 1980s, the leading paradigm (if I may quote the "classics of total synthesis") of organic synthesis was substrate-controlled stereoselectivity. Woodward himself, for example, in the synthesis of Reserpine, uses substrate-controlled diastereostereoselectivity (caused by preferred conformers of 6-membered rings in the substrate) in truly ingenious ways to stereoselevtive build up complex carbon frameworks "out of nothing". We should never forget, that organic chemistry entered a world of "luxus and decadence" when people like sharpless invented their epoxidations and dihydroxilations to easily override that substrate-generated stereocontrol with their catalysts. So to say. I have been reading a bit in this review, http://pubs.acs.org/....1021/cr000665j, about Mosher's acid, houreau's and helmchen's method for the evaluation of the absolute configuration of alcohols and amines. Interesting stuff. But it doesn't beat Xray, it seems. If you can get crystals, that is. Hm. When you only see one spot on your TLC and have two or more compounds at hand, there are still some things to try. Changing the eluent (Acetone rather than Ethyl Acetate, DCM, Ethanol, stuff like that) sometimes also (to a certain extent) can change the RF of one compound relative to the other and seperate the compounds (interestingly). And you also could try different column materials like Alox instead of silica. But it's tough, yeah. And mostly easier to change reaction conditions to avoid the side product in question. never stop when your having fun... I am still waiting for the LC/MS or GC/MS with integrated UV trace, IR spectrometer AND multidimensional, time resolved, in-system NMR spectrometer.
-
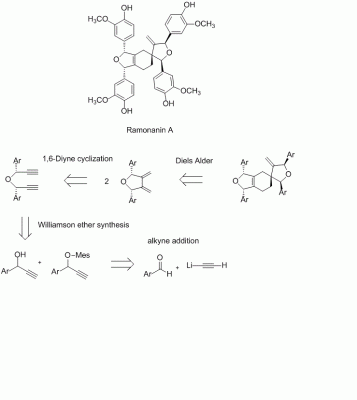
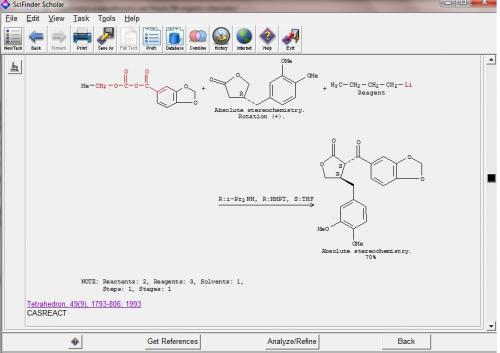
Thank you for your comprehensive remarks, hypervalent_iodine. There definitely is still a lot to discuss about, but (since this is a totaly synth faq) later, because I came over a recently isolated amazing molecule (with also interesting anti-cancer properties). [K. J. Chavez, X. Feng,J. A. Flanders E. Rodriguez, F. C. Schroeder, J. Nat. Pdt. ASAP 2011 (just look into the asaps), dx.doi.org/10.1021/np100891y] What do you think about this analysis? I'm not sure about enantioselective addition of LiC2H to an aldehyde like this. As far as I know there are enantioselective protocols for the synthesis of propargyl alcohols. And, of course, the diastereoselectivity of the Diels-Alder could be an issue. And acetylene is a gas, might be difficult to handle.
-
In that case, I would make a TLC
-

Mixed claisen condensation: diethyl carbonate w/ NaOCH2CH3
Dan_Ny replied to Genecks's topic in Organic Chemistry
Well, enolates also seem to attack the ester rather than the "carbonate" group. The nature of the nucleophile on the regioselectivity seems to be less important than the nature of the electrophile in this case.