

Dan_Ny
-
Posts
64 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Dan_Ny
-
-
It depends to what you refer. My first guess would be that you mean the stabilization of an (carb-) anion or cation. In general, though, mesomeric effect (pi-conjugation, transfer of electron density via the p- or pi- orbitals) overrides hyperconjugation (transfer of electron density from a covalent (and coplanar) bond into a empty p-orbital next to it or reverse.
Hm. I am not 100% sure about that myself. When you have a carbanion and a coplanar C-H covalent bond next to it, does the anionic electron pair transfer electron density into that covalent bond? Or does this only work reverse, in the case of a cation?
Yeah anyway. In short: pi-conjugation ("mesomeric conjugation") overrides hyperconjugation.
0 -
I totally and completely agree, titanium activatin an alkene does not sound right at all for me.
But as long as you invent a good explanation...
By the way, what exactly is the role of TiCl4 in metathesis reactions (sorry!!! I just "love 'em", like you know very well). What does it do to the first gen. grubbs?
I might ask a few questions about the diels alder as a ring-closing element in synthesis later. What we have so far is: Macrolactonization, RCM, DA. What else is there? I just want to know the best ways to close a ring...
0 -
Titanium is a reagent in this reaction and used as in excess... Not a catalyst.
0 -
6 columns. Well, i hope you are doing small-scale reactions. You were right, I had one carbon too much, apologies...
I have a new idea:
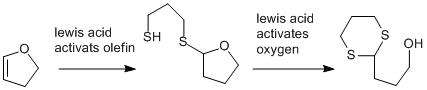
So, in my opinion, substitution at an olefin does not exist. to bypass that, what do you think about activation of the double bond by the lewis acid, attack of the first thiol group and subsequent coordination of the oxygen and attack of the second thiol group? does that sound reasonable?
Nope, we don't have a biotage, I did them all by hand...turned out to be 6 as I forgot I had one extra to do.
They use borontriflouride as the Lewis acid but pretreat the 2,3-dihydrofuran with tosic acid and methanol to get an intermediate which then undergoes the reaction with the dithiol. Maybe this will help you.
Asymmetric synthesis of the macrolide (−)-aspicilin , Guy Solladie, Inmaculada Fernandez and Carmen Maestro, Tetrahedron: Asymmetry, Volume 2, Issue 8, 1991, Pages 801-819
Yeah, that would be explained by a olefin then oxygen activation; first a protic acid for the double bond and then an oxophile. what do you think?
0 -
5 columns, hm? i hope you have access to a biotage (auto-column), then...

You're right, Horza, what I proposed does not make too much sense. But how does the Titanium activate the vinylether? I'll look through the web later, perhaps wikipedia has something to say about it...
0 -
Wow, thanks

But this is not a metathesis reaction (my mistake, i shouldn't have called it ring-opening)
0 -
I think we aren't even contradicting each other.
When I said "A suspension is material suspended in a solvent in which it is not soluble.", that also applied for oversaturated solutions. Your excess of sugar, for example, is not soluble in the solution over it due to the high amount of sugar already in it.
In the end, you can have a solution and a suspension at the same time (given mechanical force, e.g. stirring). What i suggest, however, is a careful use of both these words.
 0
0 -
we should make definitions that make sense, i suggest. a solution is a solution if a significant part of a solid is dissolved in it. a part that has chemical relevance in the sense that a reaction is meant to happen with the soluted stuff. you can always try to hairsplit any argument, but from my point of view there should be a definition and this definition should make sense and have a practical relevance.
0 -
Yeah, but my point is, that the particles are unsoluble. Otherwise, it's a solution. In a suspension, the particles never dissolve, not even partly.
0 -
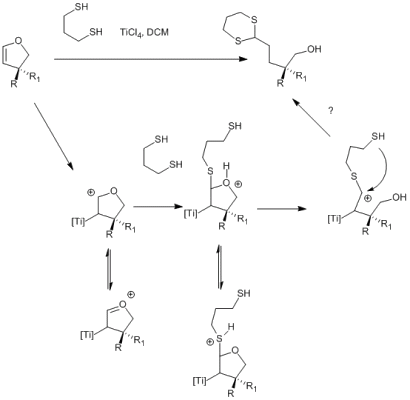
I have no idea about the mechanism of this reaction. Anybody could help?
Since according to wikipedia, TiCl4 is a Lewis, acid, we could propose this mechanism:
One drawback is, that i would not see how we get rid of the Titanium lewis-acid in the last step to furnish a reduced product. (Reductive workup perhaps?)
The second thing is that wikipedia states, that TiCl4 is an oxophiliic lewis acid. So, coordination to the oxygen in the first step in order to activate the carbon... The problem is, that lewsi activation of vinly ethers activate the carbon beta to the ether oxygen and the thiol would attack at the wrong carbon. Plus, we need double activation to get both sulfurs at the carbon.
Does anybody have an idea?
0 -
Title says it all. I have recently done some work with AgNO3 but as I handled it only for a few seconds, I didn't wear gloves and afterwards I got black stains on both of my palms. How should I remove them or should just wait for them to wear of?
In my opinion, most methods to remove elemental silver from you hands would involve removal of the hand in question, too. I would wash them very often and it'll wear off after a time...
0 -
There is a difference between a suspension and a solution.
Solution: no solid particles. All material is dissolved.
Suspension: solid particles present. Not all material dissolved.
dude this is totally INsufficient man.
A suspension is material suspended in a solvent in which it is not soluble.
The case we were talking about, my man, is an oversaturated solution (like the brine on you bench an oversaturated solution is). Because the solid IS soluble in it. And not a suspension, where the solid is'nt.
And a not oversaturated solution is that what you would call a solution.
My pleasure.
0 -
By the way, in the next step, he oxidates an enolate to a alpha-hydroxylactone under the use of an oxaziridine (an "electrophilic hydroxygroup" so to say) (see below). Enolates are known for the fact that they are configurationally unstable at the alpha-carbon. Why does he get complete stereoselectivity for hydroxylation?
The next step is something I don't completely understand. Is this a CH-activation? In a paper from 1984? But it seems to be-
What is the mechanism of it? A radical-mechanism? Hypervalent?
0 -
Dude. It's absolutely NOT save to just demand the scanned picture of an ID. Actually, I think there are also laws against private circulation of cyanides. And there have been an awful lot of cases pf people killing themselves with cyanides. I think this topic should be deleted.
0 -
I think a topic with the rare character of such an importance and impact like this one deserves further wordy elaborations. (Looking at Horza2002.)
So:
A physician, a lawyer and a Chemist discuss if it were better to have a girlfriend or to be married.
The physician states: It is better to be married. The feeling of inner safety lowers blood pressure and makes marriage healthy.
The lawyer answers: No, it certainly is better to have a grilfriend. Marriage will lead to unneccessary difficulties if one partner wants a divorce. Trust me, I have had many cases...
The Chemist: In my opininon, it is wisest to have both.
How so? The physician and the lawyer have their doubts.
Well, the Chemist explains, when my wife thinks I am with my girlfriend and my girlfriend thinks I am with my wife, I finally can have a productive time in the lab...
Ah, and I am not sure, if there is a that clear separation between a suspension and a solution.
0 -
I have my reservations about him.
I mean, if you look at his wiki article and scroll to the 2nd last paragraph (end of the Woodward-Hoffman rules), there is a clear implication that he was somehow involved in Woodward's death. Scandal!

He was also involved in Woodward's death? Hm, a dangerous person indeed. But my moral vision is beclouded by the beauty of his syntheses, I'm afraid -
0 -
 I just read this review about cross metathesis, unfortunately it did not cover too much RCM...
I just read this review about cross metathesis, unfortunately it did not cover too much RCM...Stephen J. Connon and Siegfried Blechert*, Angew. Chem. Int. Ed. 2003, 42, 1900 – 1923
I'll read yours now. You can't know enough about this. There are some interesting feautures in cross metathesis, for example chelation of the grubbs catalyst, low reactivity of electron-poor olfins, ROMP-CM cascades... cool stuff.
Ah and thanks for the review about enzymes in synthesis.
0 -
Ok, I'll draw it. might take a few minutes, though
Ah, I see it. I had considered that something along those lines may be implicated, but then I couldn't be bothered to model it in my head.
 good!
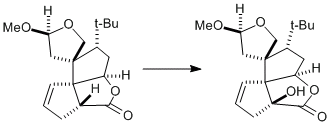
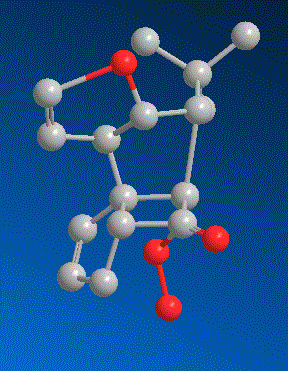
good!Well the first thing to realize is, that he used a triphenylmethylperoxide for the baeyer villiger oxidation. why would he do that?
Now, important to know is that the trityl group is one of the biggest, sterrically most demanding groups that exist.
Now look at our substrate. the only side from which this peroxide can possibly come from is from the bottom:
Now our intermediate looks like this:
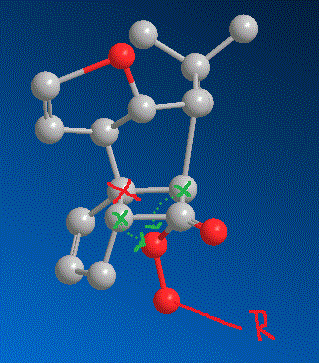
Now, look at he next picture.
The two carbons i marked green could theoretically both migrate as shown by the green arrows. But look at the carbon I marked red. It is the spiro carbon between two rings and tetrahedral. If the carbon closer to us would migrate, this tetrahedral spiro carbon would become a planar spiro carbon. Now, spiro carbons are never planar - the strain is simply too high (the electon repulsion betwee four coplanar bonds at one atom is too high). Therefore, let the other carbon, far from us, migrate. When it goes for the peroxide oxygen, the red marked carbon will be a bit deformed, but still be tetrahedral. See it? And that's why this one migrates, not the other one. Because of the spiro carbon geometry.
He is a genius, after all, this Corey...
(sorry, i did not optimize the last few structures, they look a bit weird actually - hopefully you can see it, though)
0 -
yeah youre are right, hypervalent. sorry for that, dude. (but don't you think that sometimes a laid-back attitude can be appropriate and helpful in order to keep the gravity from our science?)
0 -
Build a model. It's a steric argument.
(Why does he use tritylperoxide? Which side does it come from? And which carbon can only migrate after that and why?)
0 -
Journal of the American Chemical Society. Regurlarly.
0 -
you guys are still forgetting about the smell, dudes...
0 -
hahehahehhahahahah HAHAHAHAHAHA

 :D thx guys
:D thx guyssome of them were pretty lame, now that I think of it...
0 -
define "career" first. and "science". and, if it comes to that, "philosophy". and actually, what exactly do you mean by "useful"?
0

mesomeric and inductive effect
in Organic Chemistry
Posted
Yo
inductive effects are through-bond polarizations which are due to different electronegatvity of the two partners of a sigma bond. For example if you have a hydroxyl-group in a carbon framework, the binding electrons between C and OH will be "drawn" to the side of the hydroxy-oxygen.
mesomeric effects are polarizations vial pi-bonds. pi bonds arise from the conjugation of p orbitals. So if you have a carbonyl group, for example, in that group the oxygen draws electrons as well as by an inductive effect (sigma bond) as by an mesomeric effect (pi bond) from the carbon atom in the carbonyl.
the pi-conjugation in aromatic compounds functions (as another example of a mesomeric effect) as a "pi-relay" for electron-withdrawal via pi bonds.
yeah but you will find much better explanations of this on wikipedia:
http://en.wikipedia.org/wiki/Mesomeric_effect
Yours