

Radagast
-
Posts
32 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Radagast
-
-
Yeah, I just did this one in Math Class for our Deductive Reasoning unit. Anybody have any other interesting ones?
0 -
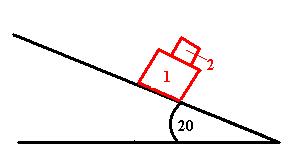
In the diagram, a person is pushing down on top of block 2 to hold blocks 1 and 2 at rest. Block 1 has a mass of 10kg and block 2 has a mass of 8kg. The coefficent of static friction between 1 and the ramp is 0.25 . When a person removes his hand...
a) Will block 1 slide down the ramp?
b) What is the minimum coefficent of static friction between block 1 and block 2 such that block 2 will not slide on block 1?
I'm having problems understanding this question, particularly part B. Any hints to send me in the right direction or explanations on the proper method to solve would be appreciated. My textbook doesn't seem to have an example quite like this one.
0 -
k, got it now. Thanks guys
 0
0 -
A chemist dissolved 25.0g of CH3COOH in enough water to make 1.0L of solution.
What is the [H+]?
What is the [CH3COOH]?
Sounds easy, but it's giving me problems (mainly because I haven't worked with organic compound questions all that much). Any hints from you guys as to what to do?
So far I have converted the 25g to moles, and when I attempted putting my answer in the Kw = [H+][OH-] equation I got the wrong answer...
0 -
I absolutely agree. A similiar question could easily be asked on my upcoming test, and now that I understand the method and have completed the work on my own, I am much more prepared.
0 -
I absolutely agree. A similiar question could easily be asked on my upcoming test, and now that I understand the method and have completed the work on my own, I am much more prepared.
0 -
Can't trust females to do a Chemistry problem eh;)
Thanks a whole lot though. My gratitude is unlimited.
0 -
Can't trust females to do a Chemistry problem eh;)
Thanks a whole lot though. My gratitude is unlimited.
0 -
Alright, I just conferred with a friend, and now were both confused lol.
Where I had this line in my calculations:
Keq = [i2] x [H2] / [HI]^2.0129 = [0.20+x] X [0.20 + x] / [(1.76+0.50)-2(0+x)]^2
She had:
.0129 = [0.20+x] X [0.20+x] / [(1.176+0.50)-2(.20+x)^2]Now we are both confused and both unsure of our methods. We don't know if once the 0.5 moles of HI is introduced, whether or not the product side will start initially at 0 or start at 0.20.
Any help sorting this one out?
0 -
Alright, I just conferred with a friend, and now were both confused lol.
Where I had this line in my calculations:
Keq = [i2] x [H2] / [HI]^2.0129 = [0.20+x] X [0.20 + x] / [(1.76+0.50)-2(0+x)]^2
She had:
.0129 = [0.20+x] X [0.20+x] / [(1.176+0.50)-2(.20+x)^2]Now we are both confused and both unsure of our methods. We don't know if once the 0.5 moles of HI is introduced, whether or not the product side will start initially at 0 or start at 0.20.
Any help sorting this one out?
0 -
What on Earth is "Keq" ? Maybe a) I'm just tired b) I don't know enough English chemistry terms. :|
Equilibrium Constant.
It's a dimensionless number that relates the concentrations in an equilbrium system and the value of the constant changes with the temperature of the system.
0 -
What on Earth is "Keq" ? Maybe a) I'm just tired b) I don't know enough English chemistry terms. :|
Equilibrium Constant.
It's a dimensionless number that relates the concentrations in an equilbrium system and the value of the constant changes with the temperature of the system.
0 -
No problem.
0 -
No problem.
0 -
Alright, I'll try it out.

Keq = [i2] x [H2] / [HI]^2
= [0.20] x [0.20] / [1.76]^2
= [0.04] / [3.0976]
= 0.0129
Now for the adding of the new concentration, that would make it:
Keq = [i2] x [H2] / [HI]^2
.0129 = [0.20+x] x [0.20 + x] / [(1.76+0.50)-2x]^2
(Taking the perfect square of that..)
0.1136 = (0.20+x) / (2.26-2x)
0.1136 (2.26-2x) = (0.20+x)
0.257 - 0.227x = 0.20 + x
0.057 = 1.227x
x = 0.0465
Therefore:
[i2] = 0.20 + .0465 = 0.2465 mole/L
[H2] = 0.20 + .0465 = 0.2465 mole/L
[HI] = 2.26 - 2(.0465) = 2.167 mole/L
Sound right?
 0
0 -
Alright, I'll try it out.

Keq = [i2] x [H2] / [HI]^2
= [0.20] x [0.20] / [1.76]^2
= [0.04] / [3.0976]
= 0.0129
Now for the adding of the new concentration, that would make it:
Keq = [i2] x [H2] / [HI]^2
.0129 = [0.20+x] x [0.20 + x] / [(1.76+0.50)-2x]^2
(Taking the perfect square of that..)
0.1136 = (0.20+x) / (2.26-2x)
0.1136 (2.26-2x) = (0.20+x)
0.257 - 0.227x = 0.20 + x
0.057 = 1.227x
x = 0.0465
Therefore:
[i2] = 0.20 + .0465 = 0.2465 mole/L
[H2] = 0.20 + .0465 = 0.2465 mole/L
[HI] = 2.26 - 2(.0465) = 2.167 mole/L
Sound right?
 0
0 -
Whoops. Type-o:p
0 -
Whoops. Type-o:p
0 -
I got a question in which I am having considerable difficulty with:
The following reaction takes place in a 1.00L vessel at 500C.
2HI(g) <---> H2(g) + I2(g)
Equilibrium concentrations were found to be 1.76 moles/L HI, 0.20 moles/L of H2, and 0.20 moles/L of I2. If an additional 0.5 moles of hydrogen iodide gas is introduced, what are the concentrations of all gases once equilibrium has again been reached?
I have tried it several times, but my Chemistry teacher said that my answers were wrong. It's due for Monday (Nov.19), so if anyone can answer it before then it would be greatly appreciated
 .0
.0 -
I got a question in which I am having considerable difficulty with:
The following reaction takes place in a 1.00L vessel at 500C.
2HI(g) <---> H2(g) + I2(g)
Equilibrium concentrations were found to be 1.76 moles/L HI, 0.20 moles/L of H2, and 0.20 moles/L of I2. If an additional 0.5 moles of hydrogen iodide gas is introduced, what are the concentrations of all gases once equilibrium has again been reached?
I have tried it several times, but my Chemistry teacher said that my answers were wrong. It's due for Monday (Nov.19), so if anyone can answer it before then it would be greatly appreciated
 .0
.0 -
-
Gibb's Free Energy is the relationship between enthalpy and entropy, to help determine whether a reaction will be spontaneous or not.
If the enthalpy is negative and entropy is positive, the reaction will always be spontaneous. With the opposite (enthalpy positive, entropy negative) the reaction will never be spontaneous.
Forumla: G = (Change in Enthalpy) - (Temperature)(Change in Entropy)
0 -
The result is expressed in terms of newtons (kilograms/meters/sec).
Isn't Newtons [ (Kilograms) x (Meters) / Seconds] ?
0 -
Wouldn't one have to use J = (Mass)(Change in Velocity) ?
Then use J = (Force) (Change in Time) ?
I can't quite remember, but thats what I thought one would do.
0

Survey For Sci Proj, Will Only Take A Min
in Genetics
Posted
1. Age? 17
2. Gender? Male
3. Where do you live? SK, Canada
4. Natural hair color? Dark Brown
5. Natural eye color? Hazel
7. Do you have colored contacts? Or what color would you prefer your eyes to be? Green
8. Heritage? Mostly Ukrainian, with a bit of German.