-
Posts
682 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Horza2002
-
-
The compound that my PhD is based on poses 10- and 12-membered carbocylic rings. In every total synthesis reported for these compounds, the ring is formed by ring closing metathesis (RCM). This method is far more efficient at forming larger membered rings than "traditional" methods, although it isn't without its problems. The size of the ring, while not as restrcited as traditional methods, still affects how efficient this reaction is. Steric factors, especially with groups bound to the alkene, also have an issue.
Another slight problem, is that Grubbs catalysts catalyse a whole range of reactions, not just ring closing metathesis. It is not underheard of to get a competing ring opening reactions and polymerisation reactions. With all that said though, metathesis is a very powerful synthetic tool.
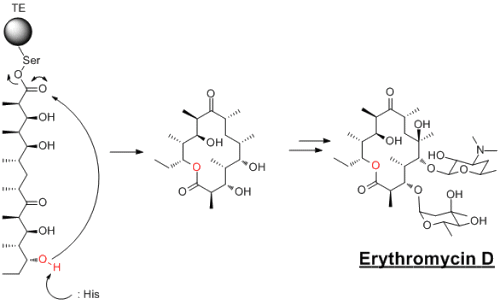
Biology, in macrocylce chemistry, is certinaly superior to anything that we chemists can currently do. There are a massive range of macrolids...the most well studied of these are the Erythromycin's. This is a PKS (polyketide synthase) natural product that conains a macrocyle that is formed when the completed polyketide chain is transfered to a thioesterase domain and cyclised. This is a very common step in biological cyclisation reactions.
Whereas Erthromycin's cylisses onto an ester, more often it is onto a thioester...hence the development of the thioester cyclisation methods. Using 2,2'-Dipyridyl sulphide and triphenylphosphine is modelled on how biology does these reactions. I have a lot of experience using (PyS)2 reaction, however im using it in a different reaction and not a macrocyclisation.
In addition to this, I think this example shows how amazing bioloigcal systems are at synthesising compounds...Erythromycin's are produced in almost complete enantioselective. Very impressive!!!
0 -
If you want any of us to expalin you the correct anwsers, then post the structures and the questions and we'll explain it you. I'm sure myself, Hypervalent and Dan_Ny will give you the help you need.
0 -
Erm, unless im not reading this correctly, it doesn't make sence. You said that the sodium hydroxide hadn't dissolved completely and then she did the titration with an acid to determine the molarity. But then you say that she leaves it a week, by which time the sodium hydroxide has completely dissolved and then you say she does the titration...so when did she do the titration, because that is important.
If she did the titration when the sodium hydroxide hadn't all dissolved, then yes she will have a molarity lower than she should. The titration only determines the concentration in solution. However, I would expect that upon addition of the acid to the solution, it would make the sodium hydroxide dissovle faster.
If she did the titration the week latter once it had all completely dissolved, then it would make no difference, she would get the right molarity.
0 -
So first of all, you will need to work out what you get if you treat each of them with nitric acid. There are 6 carbons in that structure that Dan-Ny provided and 5 of them are chiral (as you said, carbon 1 isn't chiral).
0 -
6-membered lactones are VERY stable. I have tried to hydrolyse the lactame by boiling them in 5M NaOH over the weekend....it basically just laughed at me!! It did nothing to it. Ester are fairly stable anyway and couple that with being in a stable 6-membered ring, it all makes a suprisingly stable compound. If you add groups around the ring especially on the alpha position, then it makes it even more stable! You sterically protect the carbonyl group from the hydroxide ion.
With that said, lactones can be reduced with LiAlH4... to the corrosponding 1,6-diol (you end up reducing the carboxyl section as well)
0 -
Lol thanks for that Dan_Ny
 just what I need
just what I need 
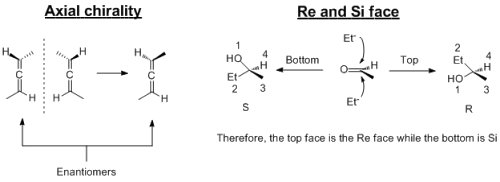
Axial chirality arises from allene (two double bonds next to each other, C=C=C). This arises because the two double bonds are orthogonal to each other because of the arrangement of the orbitals.
The Re and Si face is for prochiral molecules; these are compounds that are not chiral but if you add another group, then it will become chiral. The Re face is the face that attack leads to the R enantiomer where as the Si face leads to the S enantiomer.
I've attached a file that shows them in diagrams...I often find drawing/seeing chirality problems written down, it makes it soooo much easier than doing it in your heads.
0 -
John, I know the question was about oxygen atoms....but as you said, its extremely difficult to actually get oxygen atoms. I've just searched for water oxidation by oxygen with Web of Knowledge and there doesn't seem to be any papers on using oxygen atoms. However, there was over 10,000 results from my search so I didn't go through them all.
0 -
Dan_Ny, I know that the conformation has no influence on the R/S assignment; it is only the configuration that does. I asked that because if you have to assign the R/S centres, then you need to be able to draw the carbon with the lowest priority group facing away from you. This is much easier to do if you know the relationship between axial and equatorial substituents. That is why i said that.
This is the most common/simplest for of stereochemistry, there are other forms (like axial chirality for example). HOwever, if you are simply looking at sugars, then you will only need to know the stereogenic centre one. Even if you are not going to assign the R/S notation to each stereogenic centre, it is often simpler to use the CIP rules to see if they are indeed stereognic centres.
0 -
To see if a carbon centre is optically active, you only need to know one thing....does it have four different subtituents. If it does, it is chiral, if doesn't then its achiral.
For example, bromo-chloro-flouro methane (CHBrClF) is chiral because there are four different grous bound to the central carbon. However chloforom (CHCl3) isn't because it only have two different groups around it. If you don't need to assign the R/S notation to the stereocentres, then you don't need to know anything about chair/boat conformations.
0 -
As Hypervalent has just said, we are both PhD organic chemist...I know from my experince, then even when you know exactly what you've put into a reaction, then working out what you get at the end can sometimes be very difficult. I had one compound that took me three months to work out what it was...and that was using some very fancy and top of the range equipment and it was a very simple molecule compared to natural products. I don't mean to dishearten you about this, but I'm just trying to show you that this will be next to impossible.
Yes, you might get lucky and the firsat compound you isolate (ignoring the fact that you won't be able to isolate them without access to HPLC) but I'm sure that won't be the case. There are some labs that will anazlyse samples for you yes, but they will charge you an absolute fortune to do it...they won't be very interested in analysis samples of random people of the general public.
In addition to me being a synthetic organic chemist, I am also a microbiologist. I know how difficult it can be growing bacteria to use for feeding experiments and extracting natural products from them. Not only is it very fiddley to grow them in the first place without any contaimination, isolating the natural products takes a long time....and that is even with us overexpressing ther enzymes responsible. I say this to point out the issue that you are going to have growing enough hookworms to be isolate anything useful without contamination. How do you plan on stopping bacteria and fungi competing with the wroms for resource? Do you know which section of their life cycle the worms produce the compound your interested in? Many organisms only produce compounds at a specific time of their life...this period could last on the order of days to minutes!!
If we haven't managed to convince you that this is going to be impossibley hard, I will be more than happy to give you any help/advice that I can. I do honestly wish that this would work for you because it could be very interesting to see what happens...I just don't think its possible in a kitchen. Whatever problem you have, just post them on here and I will give you as much help as I can.
0 -
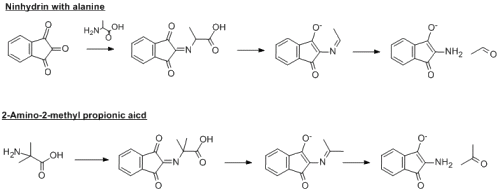
Yep I got it as valine as well. That by no means that its 100% right, but the information you were given does indicate that you started with valine. If you have any more questions, just post them on here. I'm sure there are several of us on here who will be able to help you out.
0 -
Unfortunately, it cant be 2-amino-2-methyl propionic acid (see my file). You will get acetone from this and not 2-methylpropanal. So far, you have four carbon atoms in the start material and four in the final product...there is a decarboxylation step in the mechanism and so you lose one of those carbons.
You are very close though...the NMR does indeed correlate good with 2-methylpropanal...so I would go with that being the correct aldehyde...so all you need to do is work backwards to get the amino acid....you where VERY VERY close to the correct anwser though...I'll give you a hint, its a proteinogenic amino acid.
0 -
Good good. Yes a ketal like that is a common protecting group for 1,3-diols as you have in your product. Under the reaction conditions, an internal esterification will occur because you will get a 6-membered lactone. These are very stable compounds indeed.
0 -
To make this clearer, you just need to remember that the term half-life most commonly refers to radioactive decay. But all it means is the time it takes for half you initiatial property to decrease by 50%; whether that be a radioactive atom, or compound or a protein.
As ecoli stated, diamond rearranges (im not sure decay is the right word in this case) to graphite VERY slowly and so its half-life is on the order of millions of years. The pharamceutical industry is very interested in the half-life of drug molecules. If they have a half-life too low, then they might be able to reach the site of action before its all been removed, or if its too long, it could result in adverse effects.
High temperature gas phase chemistry, in which highly unstable molecules are formed, express the stability of each of these molecules in terms of their half-life. Another example is that of sinlget oxygen...it has a half life in water of about 4milliseconds, but in organic solvents has a half-life of 16mins.
In terms of do molecules themsevles undergo radioactive decay...that remains to be seen. I beleive it is theorised that protons and neutrons (what make up the nucleus of atoms) are precited to have half-lifes on the order of the current age of the Universe...but that remains to be proven.
0 -
For some people, I think there is a physcial addiction. My parents are one example. When they go away on holiday and stop drinking umpteen cups of tea a day, they both get very bad headaches that take days to clear up if left alone. However, one cup of tea or a can of coke and the headaches are gone within the hour. Fortunatly, I don't drink tea or coffee so I don't have to put up with the same.
0 -
Sounds like a good idea to me. You borrow the money from the government, pay it back, and any interest you pay, you indirectly get the beneficts as well.
Although, I'm not sure how people would like the idea of the state reposessing their homes/goods if they couldn't make the repayments. I don't know, but I think people would be more upset about that than if a bank did....
0 -
In you molecule, the R-O-C(Me)2-O-R is the ketal. Remember than oxygen has two lone pairs of electrons (i.e. they are slightly basic in nature). Once you know that, along with having water in thre reaction, you could be able to give a mechanism. Post your ideas on here if you need some other guidence. As John Cuthber has said, they wont be interested in the acetone byproduct (HINT!).
This type of functional group is often used as a base stable protecting group for ___. Seeing as ___ is the anwser to your problem, I won't tell you just yet!
0 -
To be honest, I can't see how that would ever happen. By definition, God is supernatural where as science is the study of the natural world. It is therefore not possible to prove or disprove God using science. All science ever does is remove the need for a God (i.e. provide a natural explaintion for events orginally though to have been the result of God).
0 -
Hearts, no I wasn't suggesting they desing a new drug! I was suggesting that they look up how a specific drug works and what work is being done to improve them. Granted this still might be a little to advanced.
0 -
If you'd like a little more detail, then we can discuss the mechanism of combutions.
Combustion is generally accepted to be radical chain reaction mechanism; although it is not completely understood. The initiation is forming an oxygen radical from molecular oxygen which then abstracts a hydrogen to leave a hydrogen peroxide radical. This radical abstracts a second hydrogen from the fuel to give hydrogen peroxide, which then homolyses to give two hydroxyl radicals. These are very reactive species and then go onto abstract further hydrogens (to give water) and oxides the carbon atoms (to give carbon dioxide).
The obvious question here is where does the initial oxygen radical come from? The most accepted idea is that molecular oxygen (a triplet in its ground state) is converted to singlet oxygen (by flipping the spin of one of the unpaired electrons in molecular oxygen). To do this, a relatively large amount of energy is required because singlet oxygen is not very stable. This energy normally comes from whatever ignites the fuel (a spark, another flame, etc).
0 -
There are some isomerisation reactions that are exothermic. For example, a carbonyl is more stable than the corrosponding enol form....so when a compound isomerises from the enol to the carbonyl compound, heat will be released and therefore be exothermic.
0 -
If your planning on doing this at home, then heres some advice.....don't bother! It will be impossible.
Without access to HPLC, chiral HPLC, high resolution MS, high-field NMR and IR instrucments, you will not be able to work out the structure of the compound. It often takes years to determine the absolute structure of a new natural product.
Another major problem is isolating the product in the first place. While a hook worm type creature is relatively simple, it will still produce thousands, if not tens of thousands, of different compounds. What are the benficail effects you think they cause? Because the only way your going to know which of the potential thousand or so compounds you'll isolate, you will have the test them all to see which ones give you the desired effect. Without HPLC, it will be impossible to seperate the mixture. And if your planning on doing this on yourself, which in itself is a very bad idea, you will need lots and lots of each of the compounds.
0 -
To anwser this, I am assuming you mean the act of combustion and not the flame itself.
Burnign is, at the simplest level, a chemical reaction; one set of chemical bonds are replaced by another set (normally stronger). For a reaction to occur, the change in Gibbs free energy (G) should be negative. Since G=H-TS, for a given temperature, an exothermic reaction with an increase in the number of molecules will be very favourable.
The majority of fuels have lots of carbon-carbon and carbon-hydrogen bonds (e.g. petrol, wax, wood, etc.) in them. These bonds are relatively strong, however, carbon-oxygen bonds are stronger. When the fuel is burnt, these bonds are replaced with carbon-oxygen double bonds and hydrogen-oxygen bonds. These bonds are stronger than the starting bonds and so there is an enthalpic gain when they are replaced.
Carbon-Carbon = 347 kJmol-1
Carbon-hydrogen = 435 kJmol-1
Oxygen-carbon double = 805 kJmol-1
Oxygen-hydrogen = 464kJmol-1
Oxygen-oxygen = 498kJmol-1
So for example, burning pentane, the equation is
C5H12 + 8O2 ==> 5CO2 + 6H2O
The enthalpy of the starting material is:
5(C-C) + 12(C-H) + 8(O=O)
5(347) + 12(435) + 8(498) = 10939kJmol-1
The enthalpy of the products:
10(C=O) + 12(O-H)
5(805) + 12(464) = 9593kJmol-1
Therefore the change in enthalpy of this reaction is 9593-10939 = -1346kJmol-1 (i.e. its exothermic and favourable).
Another favourable aspect to consider is that there is an increase in entropy. There are 9 starting material molecules and 11 product molecules...so overal, burning a fuel has a negative change in Gibbs energy and so is favourable.
Thats probably a little more indepth than you wanted but o well!
0 -
Yes it will work, a small amount of the enolate will be in solution to allow the reaction to occur.
0



Chirality of a carbohydrate
in Organic Chemistry
Posted
Just to pouint out, there are a few other types of chirality that exist. One of them is helical chirality; which way the helix is cooled up. The most common form of this is DNA. In almost all cells, DNA exists it the B-DNA form. Among other difference, the helix is wound up "right handed"...there also exists another form of DNA called Z-DNA in which the coil is wound up "left-handed".
Yes, sugar stereochemsitry is very complicated because one of the stereocentres only exists in the cyclic form. Incidently, the aldehyde in the straight chain form is prochiral. Therefore the two faces of the aldheyde (top and bottom) will be Re and Si....which enatiomer you get depends on which side the hydroxy group attacks the carbonyl. In solution, there is actually a mixture of the two epimers at that centre.
As Dan_Ny has said, for this problem, using nitric acid is kinda like going after a fly with a bazooka. It is very strong and in practise would tare the sugar to pieces, you'll get a mixture of elimination, epoxidation, polymerisation and racemisation reactions all going.