-
Posts
682 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Horza2002
-
-
This reaction will be very difficult to do. The problem arises because the initally formed monoalkylated product is more nucleophilic that the start material (due to the alkly chain electron donating properties). What you are probably ending up with is the quaternary nitrogen salts resulting from another subsitution of the alkly iodide.
If I were you, I would stop trying to do this and try a reductive amination (using the corrossonding aldehyde and sodium cyanoborohydride) and the monoprotected piperazine. That way you will only get the product you want without any over alkyation reactions.
0 -
...it is not a nitrating agent at all. For one thing, there is no nitro group in it! Sulphuric acid is used to protonate nitric acid which then decomposes to NO2+ which is the actual nitrating species. And I really wouldn't call is the "best". Ever tried nitrating anything with this method? You end up with a mixture of all possible regioisomers that are often next to impossible to seperate.
1 -
Hi there,
I think there is an inherant problem with this reaction. When undergraduates are taught about making amines, the first thing you are told is that you can't make them from alkyating a NH2...the monoalkylated nitrogen is more reactive than the unalkylated nitrogen due to the electron donating properties of the alkly chain. I would expected that you would eventually end up the the tetraalkylated amine instead of your desired monoalkayted.
Additionally, if you add to strong a base to this reaction, you will deprotonate the Boc N-H (it is rather acidic due to the electron withdrawing properties of the adjacent carbonyl). If that happens, then you are going to end up alkyating the wrong nitrogen atom.
You could potentially make the dianion (deprotonate the Boc nitrogen and the NH2) with two equivalents of BuLi and then try adding one equiv of the iodide, but I expect you will get cylisation and a mixture of products.
The main issue with this reaction is the diiodide...is it essential that you do this? If not, then I would suggest adding a few more steps into the scheme and introduce the second iodide after you have alkylated the hydrazine
0 -
Think about how many resonance structure you can draw for the C-4 and C-5 anions. How might this effect the stability?
0 -
Yes it will work because I have done this in our lab. We have done it on a number of enzymes and a number of natural product analogues to aid in their purification. As in the link, a click reaction is usually used to introduce the biotin linker to the substrate/enzyme of interest as it is very easy to do.
There are several limitations to this procedure though. Binding of the biotinaylted substrate is not always easy. We found that the larger the substrate you try to trap, the harder it is to bind. In addition to this, you need to use alot of biotin to elute your trapped substrate from the strepavidine column. Commerical biotin is really really expensive at the moment and so doing this on a large scale is not really practical. It is possible to overproduce biotin from microorganism and purify it, but this is a very lengthy and time consuming process.
1 -
-
1,2-Dimethylpropane is the same as 2-methyl butane.
2-Methyl butane is the correct name since you name some from the longest chain of carbons in the molecule (in this case its 4 so you use butane)
2 -
I'm not sure that storing it in ether for a long period of time will be a good idea. As CharonY has stated, the ether is probably going to alter the fold over time. Why can't you just store it in 25% glycerol suspension like normal?
0 -
... and I think the topic is a little too broad to explain here.
Can I nominate this for understatement of the year please?
 0
0 -
Out ouf curiosity, why are you trying to make the sulphonamide that way? Surely it would be much easier to react the R-NH2 (amine) with tosyl chloride to give you the sulphonamide. I know this works great because I have actually just done the reaction 15 mins ago
 0
0 -
By methyal i guess he means methanal and ethanal...
0 -
You've posted this in the wrong section. This is about organic chemistry not nuclear physics...
0 -
We have cellophane discs in our lab which we use to grow Streptomyces bacteria on so they don't grow into the agar. This allows all small molecules to pass through them, but not bacteria cells. Any good?
0 -
-
Thanks Hyper for rewording my post....
0 -
No, I wouldn't say everyone knows where there going...loads of undergrads here when you ask where that bond comes from have no idea...they just draw it.
And its not really about being pedantic...its about being accurate
0 -
I think thats a mistake. A to D is fine, and OsO4 is a well known reagent for the formation of cis-1,2-diols...and D and E are isomers of each other so thats fine (do you know what type of isomers they are of each other?). However, you don't get the diketone product, you only get the diol.
0 -
Try typing "feul isomeristion" into google...and go read the 1,970,000 pages that come up a result...
0 -
...theres already a thread with these exact same questions on:
http://www.scienceforums.net/topic/61949-mechanisms-help/
I take it you two are at the sam University then
0 -
How are you making it? IOn my experience, its easier to avoid getting impurities in your sample than trying to get them out again.
0 -
The last one is an ozonolysis and looks fine to me
0 -
Tut tut, I would expect better from someone like you to be honest...
0 -
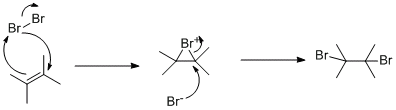
Hyper, the mechanism for the formation of the bromonium ion is wrong...you need another curly arrow from the bomrin thats being attacked lone pair to the other end of the alkene
0 -
If all you've put in is bromine, carbon tetrachloride and your start material, that would lead me to assume that it is actually a di-bromination....as you said, there is no hydrogen to come form anywhere.
0



Synthesis of 2,3-dihydroindole
in Homework Help
Posted
The final step can be done, but you need to add some other reagents in there. What is the inital product when an amine reacts with a carbonyl?
Additionally, there is a possible alteration of your method that will get you from the 2-amino toluene (number 2) all the way to your benzyl alcohol (number 5) in a single step. Think about the position you are trying to oxidise and what reagents are out there to do this transformation (HINT: its a p-block element based reagent)