-
Posts
286 -
Joined
-
Last visited
-
Days Won
1
Content Type
Profiles
Forums
Events
Posts posted by NTuft
-
-
16 minutes ago, exchemist said:
This is all about the behaviour of electrons in atoms and molecules, not the nuclei.
Au contraire, mon ami -- the two are inexorably linked I think we would agree, and the Moon model posits tandems of nucleons at discrete points on a definite structure, as I understand it. An analogy would be crystal structure. I do not understand it, and need to read and study more.
16 minutes ago, exchemist said:Hydrogen bonding remains I think something of an enigma. At one time there was a view that it was just a special case of an attraction between permanent dipoles, but in fact it has directional character, which seems to involve the electrons of the "lone pairs" of electrons on the electronegative [ed.:electronegativity deserves emphasis to the question at hand] atom. So there seems to be an element of electron pair sharing, as in a covalent bond.
Hydrogen does seem to be acting funny, but it doesn't have much of a shell now, does it?.. It is like an iota. The table is set with many and various names from the lexicon but some see it as iterations on an iota, or an iota that is paired and by any measure significant. I have not processed what you've actually written and may be responding mechanically. -1 me to 0.
0 -
2 hours ago, nae said:
Can someone explain Van Der Waal/London forces to me as simple as possible? Along with explaining Permanent dipole-dipole forces?
I have a hard time understanding the intermolecular forces except hydrogen bonding but the rest is just like...???
1 hour ago, exchemist said:[...]The name Van der Waals forces is given to all intermolecular attractions that don't involve a chemical bond. So the term includes both London (dispersion) forces and the attraction between permanent dipoles. (But it would not include hydrogen bonds, as these have some directional bonding character and are thus not entirely electrostatic dipole attractions.)
13 minutes ago, MigL said:Simplest explanation possble ...
Residual Electromagnetic forces.J. Diderik van der Waals's "inter-molecular" forces explanation is distance-dependent, and are "comparatively weak, and vanish at longer distances", theoretically.
QuoteThe van der Waals forces are usually described as a combination of the London dispersion forces between "instantaneously induced dipoles", Debye forces between permanent dipoles and induced dipoles, and the Keesom force between permanent molecular dipoles whose rotational orientations are dynamically averaged over time.
To complicate the matter further, I ask: are we dealing with nuclear droplets, or have we, "flown to the Moon" model, of nuclear structure? I for one do not understand hydrogen bonding, either, and need to review the basics.
+1 for exchemist's explanation, and I agree with MigL there is not much to add to address the question properly (caveat: Again, I need to review even these basics). In my opinion the better formulation of Occam's razor states that the explanation needs to be as complicated as needs be dictated by the nature of the question posed...
1 -
I have always thought of there being parentheses inside the root exponential function.
I would argue that the numbers are opposite in magnitude but are the same number, precisely.
It may be that I was only trying to find the unit hyperbola, or the complex hyperbolic space.
QuoteParametrization:
A direct way to parameterizing the unit hyperbola starts with the hyperbola xy = 1 parameterized with the exponential function:
.
This hyperbola is transformed into the unit hyperbola by a linear mapping having the matrix:
:
This parameter t is the hyperbolic angle, which is the argument of the hyperbolic functions.
One finds an early expression of the parametrized unit hyperbola in Elements of Dynamic (1878) by W. K. Clifford. He describes quasi-harmonic motion in a hyperbola as follows:
QuoteThe motion
has some curious analogies to elliptic harmonic motion. ... The acceleration
thus it is always proportional to the distance from the centre, as in elliptic harmonic motion, but directed away from the centre.
I thought perhaps the vertical (lower, or limit) bounds for the complex graph should be
, and the horizontal perhaps
when
, which would make an ellipse, so the unit hyperbola scaled differently and now with an ellipse...
but of course we can not screw up hyperbolic functions:
Quoteis half the difference of
and
is the average of
and
It may be we can already do everything we need to with
, but it seems clunky to me...
I doubt this is correct or useful either but I had it worked out we could quantize Kinetic Energy with the bound that makes .5 from the function,
, or -- set that limit velocity as equal to the mass of the proton.
I had Potential Energy for, I imagine, an electron
whereby two electrons at (-.25) balance a proton charge (possibly confounding charges and energies...), but, with TI-83 (handheld scientific calculator) I had the P.E. function increasingly negatively (as though the electron were further from the proton), whereas the KE function
set an upper .5 bound with
and was going towards a limit of 0. I cannot re-create the numbers the same for PE through WolframAlpha computation, and I'm not sure what the exponent should be for PE (3/2, 1.5,...).
I will try to only "crank" out what is necessary for this thread. I would not rule out that
On 5/11/2022 at 2:20 AM, studiot said:There is no problem with defining 'square roots'.
I thought the "imaginary unit" was kind of clunky, and we could somehow give magnitude to complex numbers if we define the i as being members of set. But, maybe we can do everything with i being the multiplier that signifies a negative root.
Lastly I don't know if I can use +1 and -1 as arguments in the function given current definitions, but iterations of +1's and -1/2's for protons and electrons respectively I thought made sense some how.
0 -
6 hours ago, studiot said:
The square root of something is defined as that which which when multiplied by itself yields that very something.
There is a problem with defining the square roots of negative numbers, in my opinion. I went over how I wanted to do that. There are two ways to do it for positive numbers, and I think there are two ways to do it for negative numbers.
Also, the explanation on the domain set being mapped onto the codomain by a function, and the many-to-one vs. one-to-many rules are helpful, thank you. I will try to wrap my head around it... Sorry, but I think it will help.
4 hours ago, uncool said:…no, NTuft, that is not why e^(i pi) = -1. Your blind substitution is not correct.
You are claiming that e^(x*i*pi) = x cos(pi) + i x sin(pi) = -x, if I’ve remade your equations correctly. This simply isn’t true. It has nothing to do with the actual justification of the original equation (which has to do with McLaurin series), and is self-contradictory with a bit of thought.
Hi uncool.
I do not think you have what I am claiming figured. I meant to illustrate in the post I quoted you in above that the numbers you ran on WA were not what I was implying. In the second iteration, still yet, no -- operative is: "with
". Then the imaginary number in the exponent is now that one I've just set, which I then split.
I will definitely look into how the McLaurin series is tied in and I appreciate your knowledge about (I imagine) sequences, series(just mentioned), sets (I know), and the postulates, forms, rules and proofs that go into that branch of math, which I bet you know well -- it's not my strong suit.
--
"e^(x*i*pi) = x cos(pi) + i x sin(pi) = -x,"
x=pi already in that equation, no?
0 -
2 hours ago, uncool said:
…none of this is accurate, and this can be checked directly by e.g. wolfram alpha.
e^(i’ pi) = e^ (i sqrt(2) pi) = cos(sqrt(2) pi) + i sin(sqrt(2) pi) ~ -0.266 - 0.963 i
when
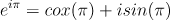 =
= 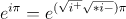 =
= 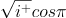 +
+ 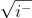
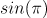 = (+1)([cos(pi)=-1]) + (-1)([sin(pi)=0]) = (+1)(-1) + (-1)(0) = -1
= (+1)([cos(pi)=-1]) + (-1)([sin(pi)=0]) = (+1)(-1) + (-1)(0) = -1
I think the positive co-efficient (+1) is there in front of cosine, though i+ is not denoted--since it's the "real" half of the root of -1
-----
using
now with
= (i'+)(i'-)=(+1.4142...)(-1.4142...),
(i'+)cos(
)+ (i'-)sin(
) =
cos(
)+
sin(
)=(
)(-1) + (
)(0) = -
;
since that co-efficient in front of cosine is now
0 -
-
-
23 minutes ago, exchemist said:
I find the concept of oxidation states helps. The higher the +ve number, the more oxidised the species, which signifies the removal (whether real or notional) of more electrons.
+ve number?
I think I understand the same. Higher positive number meaning net charge higher due to fewer electrons (-) in the balance.
0 -
On 5/8/2022 at 11:13 PM, chenbeier said:
Why not?
Alkali or earth alkali sulfates can only reduced with aluminium, calcium, sodium and others.
On 5/9/2022 at 1:23 AM, exchemist said:e.g. Mn (II).
I can't always keep it straight myself, so:
Oxidizing agent:
https://handwiki.org/wiki/Chemistry:Oxidizing agent
QuoteAn oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from an reducing agent (called the reductant, reducer, or electron donor). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the [Ed.:error here:
increases] [reductant] increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combustion, many explosives, and organic redox reactions involve atom-transfer reactions.
https://handwiki.org/wiki/Chemistry:Reducing agent
Reducing agent:
QuoteA reducing agent (also known as a reductant, reducer, or electron donor) is an element or compound in a redox chemical reaction that loses or "donates" an electron to an electron recipient (called the oxidizing agent, oxidant, oxidizer, or electron acceptor). In other words, a reducer is any substance that reduces another substance. The oxidation state, which describes the degree of loss of electrons, of the reducer increases while that of the oxidizer decreases; this is expressed by saying that reducers "undergo oxidation" and "are oxidized" while oxidizers "undergo reduction" and "are reduced". Thus, reducing agents "reduce" oxidizers by reducing (decreasing) their oxidation state while oxidizing agents "oxidize" reducers by increasing their oxidation state.
To clarify, in a redox reaction, the agent whose oxidation state increases, that "loses/donates electrons", that "is oxidized", and that "reduces" is called the reducer or reducing agent, while the agent whose oxidation state decreases, that "gains/accepts/receives electrons", that "is reduced", and that "oxidizes" is called the oxidizer or oxidizing agent. A reducing agent is thus oxidized by an oxidizer when it loses electrons that are gained by this oxidizing agent; this oxidizing agent is itself simultaneously reduced by the reducer.
In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). A reducing agent typically is in one of its lower possible oxidation states and is known as the electron donor. Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. For example, consider the overall reaction for aerobic cellular respiration:
- C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l)
The oxygen (O2) is being reduced, so it is the oxidizing agent. The glucose (C6H12O6) is being oxidized, so it is the reducing agent.
In organic chemistry, reduction usually refers to the addition of hydrogen to a molecule, though the aforementioned definition still applies. For example, the oxidizing agent benzene is reduced to cyclohexane in the presence of a platinum catalyst:
- C6H6 + 3 H2 → C6H12
Historically, reduction referred to the removal of oxygen from a compound, hence the name 'reduction'. An important example of this phenomenon occurred during the Great Oxidation Event, in which biologically−produced molecular oxygen (dioxygen (O2), an oxidizer and electron recipient) was added to the early Earth's atmosphere, which was originally a weakly reducing atmosphere containing reducing gases like methane (CH4) and carbon monoxide (CO) (along with other electron donors) and practically no oxygen because any that was produced would react with these or other reducers (particularly with iron dissolved in sea water), resulting in their removal. By using water as a reducing agent, aquatic photosynthesizing cyanobacteria produced this molecular oxygen as a waste product.[2] This O2 initially oxidized the ocean's dissolved ferrous iron (Fe(II) − meaning iron in its +2 oxidation state) to form insoluble ferric iron oxides such as Iron(III) oxide (Fe(II) lost an electron to the oxidizer and became Fe(III) − meaning iron in its +3 oxidation state) that precipitated down to the ocean floor to form banded iron formations, thereby removing the oxygen (and the iron) [Ed.: stories! lies! alcoa! They hid a bunch of Davy and Faraday's stuff, I bet. They were galvanizing boat hulls w electrolysis ffs. bwahaha...]. The rate of production of oxygen eventually exceeded the availability of reducing materials that removed oxygen, which ultimately led Earth to gain a strongly oxidizing atmosphere containing abundant oxygen (like the modern atmosphere).[3] The modern sense of donating electrons is a generalization of this idea, acknowledging that other components can play a similar chemical role to oxygen.
0 -
(+)(+)
(-)(-)
(+)(-)
(-)(+)
0 -
20 hours ago, chenbeier said:
Why not?
Alkali or earth alkali sulfates can only reduced with aluminium, calcium, sodium and others.
Al uminum! 🤣
j/k. we'll defer to Humphry Davy 's choice about that.
0 -
Quote
You can use sulphates as oxidisers, if the fuel is a strong enough reductant.
That goes contrary to my understanding of oxidizers and reducers, John.
Will they spontaneously react, as Sodium and Chlorine, Nitric acid and Hydrazine, or even Lead and Sulphuric acid ?
Or must an initial energy be added, as in your posted video, to get past the potential barrier ?Please explain the reaction process.
( my last Chem course was Gr.13 in 1976-77 )Are we talking about anhydrous sulfates? What ones? What is a strong enough reductant? Don't say aluminium.
0 -
1 hour ago, exchemist said:
Enlighten me.
We're going off-topic, but, it looks like a job by:
https://handwiki.org/wiki/Biology:Dissimilatory metal-reducing microorganisms
QuoteDissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.[1] The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well.[2][3][4] But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].[1]
something weird about their cytochrome c's... CymA and TorC genes or something.
0 -
Unbelievable overtake.
Thanks, I hadn't seen this.
0 -
8 hours ago, exchemist said:
There are micro-organisms that use sulphate to oxidise carbohydrates or hydrogen to obtain energy for their metabolism, so there are circumstances in which ΔG is -ve for a reaction scheme in which it behaves as an oxidiser, being reduced to H2S in the process.
"Eaters of rock"...
Ever heard/read about the polymetallic nodules on the ocean/sea floor? I don't think they precipitated or accreted over time randomly.
0 -
Quote
"f(x,y,z) = volume of the rectangular paralelpiped=x*y*z where x=length(l}, y=width(w), z=height(h) of rectangular parallelpiped"
x,y,z is given to you as terms that define the ellipsoid.
How about you try to define the volume.. centered.. within what is given -- the ellipsoid?
0 -
set: https://handwiki.org/wiki/Set_(mathematics)
QuoteIn mathematics, a set is a collection of elements.[1][2][3] The elements that make up a set can be any kind of mathematical objects: numbers, symbols, points in space, lines, other geometrical shapes, variables, or even other sets.[4] The set with no element is the empty set; a set with a single element is a singleton. A set may have a finite number of elements or be an infinite set. Two sets are equal if and only if they have precisely the same elements.
derivative: https://handwiki.org/wiki/Derivative
Quote[...]the derivative is often described as the "instantaneous rate of change", the ratio of the instantaneous change in the dependent variable to that of the independent variable.
differential: https://handwiki.org/wiki/Differential_(mathematics)
QuoteIn mathematics, differential refers to infinitesimal differences or to the derivatives of functions.
function: https://handwiki.org/wiki/Function_(mathematics)
QuoteIn mathematics, a function is a binary relation between two sets that associates each element of the first set to exactly one element of the second set. Typical examples are functions from integers to integers, or from the real numbers to real numbers.
differential operator: https://handwiki.org/wiki/Differential_operator
QuoteIn mathematics, a differential operator is an operator defined as a function of the differentiation operator. It is helpful, as a matter of notation first, to consider differentiation as an abstract operation that accepts a function and returns another function (in the style of a higher-order function in computer science).
it'd seem to establish it as a function would only require that the set be mapped to another set. as a function it could then be differentiated, producing the derivatives as proposed. what this entails, i do not know -- it could be a nothing burger.
or, it could be a Hamiltonian operator: the potential number of values that can be assigned to objects (particles) as kinetic(i'') or potential(i''') energy are unlimited, and it seems to me a 'gapped hamiltonian' in that the values are discrete with 'jumps' in between. just more speculations.
0 -
(OIL-RIG) Oxidation Is Loss, Reduction Is Gain [of electrons]
0 -
Dear @studiot,
On 5/3/2022 at 4:20 AM, studiot said:Please elaborate on what you mean by these statements, since they are not mathematical ones I recognise.
Note mathematical statements need not be symbolic, English is perfectly good.
Please pay particular attention to what you mean by 'derivation' and 'set'.
From page 1:
QuoteI understand a derivative to be a measure of infinitesimal change at a point. [...]
I think that this is the purpose of set theory: numbers are what we commonly refer to as the collection of objects [...]
These are my working definitions, and I know they're not correct, so I will look to redefine these terms properly, but, please do impart directly what you think is the most correct or necessary conditions/definitions for the purpose here, if you see fit.
Quotesince they are not mathematical ones I recognise.
I am not able to make LaTeX work properly yet, but I'm reading through that information.
A correction may help you recognize the operation :
I apologize, i do take this seriously. And no, I cannot define all my terms properly, I'll work on it. Your participation is appreciated but is up to you.
Thanks
0 -
On 5/3/2022 at 10:12 PM, Dhamnekar Win,odd said:On 5/3/2022 at 10:04 AM, Dhamnekar Win,odd said:
Now, I rewrite the corrected balanced reaction 2VO2+ + Zn0 + 4H+ ⇌ 2VO2+ + Zn2+ + 2H2O
If I write the balanced equation as 2VO2+ + Zn0 + 2H+ ⇌ 2VO2+ + Zn2+O-2 + H2O
Is this correct?
I do not know.
Perhaps split apart the reduction from the oxidation reaction.
What is oxidized, what is reduced? Is the start => change of the solution set-up to allow this reaction?
Remember they want the E°.
0 -
10 hours ago, KickMePlease said:
maybe in Us where the govermnet has more respect for peoples
The grass is soaked in glyphosate.
We do not have a representative government, I don't think anyone in the West does. Money's influence on political campaigns here runs the show.
As regards the detail from your original post, I don't think the specifics you give are germane to the question. This post quoted here meanders a ways, too.
0 -
6 hours ago, studiot said:
I seen no point worrying about the rest of the material in your post until the basics are cleared up.
Use the fundamental theorem of arithmetic to make the top-down set.
2 hours ago, studiot said:If you are not going to take the discussion seriously, you will need to find another to continue the discussion with.
I will try to formulate it properly. In this case I'm using it as the operation whereby the power (dimension) is reduced by 1 and that is brought down to a co-efficient.
0 -
A standard technique in editorialization is to insert [Ed: ]
How can you operate latex on this site, or where would you recommend I find it, please.
0 -


Can someone explain Van Der Waal/London forces to me as simple as possible? Along with explaining Permanent dipole-dipole forces?
in Chemistry
Posted
My point of contention would be that an extended nuclear structure is what makes possible the exposure of a positive dipole, and that this does play a role in nucleophilic-electrophilic bonding, for example. That said, I'm well far off from what should be addressed here so my apologies for that.