

King E
-
Posts
56 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by King E
-
-
17 hours ago, swansont said:
Dipoles in the material will align with the electric field
??
0 -
-
I learned from a source that when external magnetic field is applied, parallel spin/spin up has lower energy than spin down. But why? How does this relate to two like poles of magnets facing each other and two unlike poles facing each other?
0 -
7 hours ago, Asheekay said:
Oxidation states have a lot to do with the electronegativity value of an element, regardless of whether the compound is covalent or ionic.
For example, in your case of $H_2S$, hydrogen has an electronegativity of 2.20 while sulphur has a value of 2.58, being slightly more electronegative than hydrogen.
Hence in hydrogen sulphide the shared electron pair spends slightly more time with sulphur than they do with hydrogen. That is why hydrogen gets a partial negative charge while sulphur gets a partial positive charge.
What about in case of O₂ ? Or H₂ ? The electronegativity is same. Which atom will get partial negative charge and which will get partial positive charge in the above mentioned cases?
15 hours ago, OldChemE said:Oxidation states relate to the electron structure of the given atom, generally taking into account the octet rule. The Octet rule basically says that atoms "prefer" to have 8 electrons in their outer shells (there are exceptions and it is not very rigorous-- but it works). The outer shells of Sulfur are 3s2 3p4, for a total of 6 electrons. By 'sharing' 2 more electrons (from the hydrogen atoms) the atom fulfills the octet rule, but that gives it an electric charge of -2 (2 extra electrons). As these two electrons are from the two Hydrogen atoms, they are now are each +1. The extent to which the electrons are only shared, as opposed to one atom taking and varies. There is a lot more technical detail to that. You should note that the arrangement of the Periodic Table is ties into this, as elements that usually have the same oxidation states are all in the same column.
Each hydrogen atom in H2S , no doubt gives 1 electron to sulphur but also takes 1 electron from sulphur. Similarly the sulphur atom takes 1 electron from each hydrogen atom but also gives 1 electron to each hydrogen atom. So why isn't the oxidation state of each hydrogen atom -1 and that of sulphur +2 in H2S ?
0 -
How is the oxidation state in covalent compounds calculated ? For example, in H₂S, why does hydrogen have an oxidation state of +1 and sulphur has oxidation state of -2 as the electrons are being shared ? Can hydrogen have oxidation state of -1 and sulphur +2 (because the electrons are shared) ?
0 -
16 minutes ago, chenbeier said:
No, the full k shell is helium structure and very stable. So there can no additional electron to L, the same reason you can not make a He- .
If you bump an electron to H- ion, can't the electron just enter the empty L shell by releasing energy?
0 -
14 minutes ago, chenbeier said:
How many electrons does hydrogen have? How can the K shell be full
What I meant to say is can a Hydrogen -2 ion be formed?
0 -
In hydrogen, why cannot electrons enter an empty shell after the K shell is filled?
0 -
13 hours ago, Bufofrog said:
What kind of interaction? You do know you can Google the answer in about 5 seconds?
I did. Some people are saying that its a physical change and some are saying that it is a chemical change.
11 hours ago, studiot said:New (chemical) species are formed.
In a solution the constituents exhibit individual chemical characteristics. You are saying that new chemical species are formed. So will the "NaCl in water " solution have different chemical properties from its constituents(NaCl and H2O)? i.e. is a "NaCl in water" solution a new compound with respect to NaCl or H2O ? And is the ion surrounded by water molecules (solvation shell) a new compound?
0 -
1 hour ago, Bufofrog said:
Sounds like homework.
What makes the Na+ and Cl- come apart?
It ain’t.
The interaction of water molecules.0 -
9 minutes ago, studiot said:
Yes
How?
0 -
Is the solvation of salt (NaCl) in water a chemical reaction?
0 -
On 8/22/2020 at 6:54 PM, Strange said:
To give an even simpler answer: they get to the surface and then escape!
That may be too simple to be useful. But as you add heat from the bottom, it will cause convection which brings those molecules up to the surface. Also, as the fastest molecules at the surface escape, the average kinetic energy of the top layer decreases; in other words it cools and so will sink lower in the container.
Why doesn’t that happen by conduction, when the molecules below vibrate upon heating and collide with each other they transfer energy all the way through other molecules to the surface molecules?
0 -
I heard, molecules only at the surface of the liquid evaporate. But do the molecules below the surface of the liquid evaporate? Suppose we heat a container containing a liquid from the bottom. So molecules at the bottom of the container will have higher kinetic energy than the molecules on the surface. How will the molecules on the bottom escape as vapours?
1 -
If all the molecules at the same height in a container feel the same pressure then how does it remain true if the shape of the container is changed? Where are the vertical reaction vectors coming from on the right side, given that the container is a single rigid body and is thus not being pushed down onto the liquid molecules by gravity?0
-
11 minutes ago, studiot said:
I agree that the OP definition is not quite right, but both your A and B examples have two errors.
Yes a zero error is a systematic error but it is not due to a wrongly marked or non uniform graduation and yes it can result in an increased or decreased actual measurement.
A simple example of a zero error would be dirt on the pan of an otherwise accurate weighing scale.
So “The error which occurs in a measuring instrument due to non-leveling of scales on zero point is known as zero error”?
0 -
Is this the correct definition of zero error?
The systematic error in a measuring instrument due to non-uniform or wrongly marked graduation due to which a measurement may be less or greater than actual measurement is called zero error of the measuring instrument.
0 -
Just now, Strange said:
Normally centimetres and millimetres. What would you expect?
Some are marked in inches as well. Are they standard as well?
0 -
In other words what is the scale of Standard Metre Rule?
0 -
1 minute ago, Strange said:
Uh, OK.
Did this answer help more:
(I thought it was spot on.)
Yes it helped and I got my answer.
2 -
27 minutes ago, Strange said:50 minutes ago, studiot said:27 minutes ago, Strange said:
Can you explain why not? Or ask your question more clearly
Yes because you find faults in the question whenever your answer doesn't help.
0 -
1 minute ago, Strange said:
This illustration of sine and cosine curves might help: https://www.mathsisfun.com/algebra/trig-sin-cos-tan-graphs.html
I know that but they don't answer my question.
0 -
-
8 minutes ago, joigus said:
So the object passes through the rest but isn’t continuously at rest because of the force of gravity. This obeys Newton’s law doesn’t it?
0

.thumb.png.84d6c1b437d992a62e66c7e392d8d247.png)
.thumb.png.33c3ee6bdb5c5e23bb557d5ef55761b0.png)

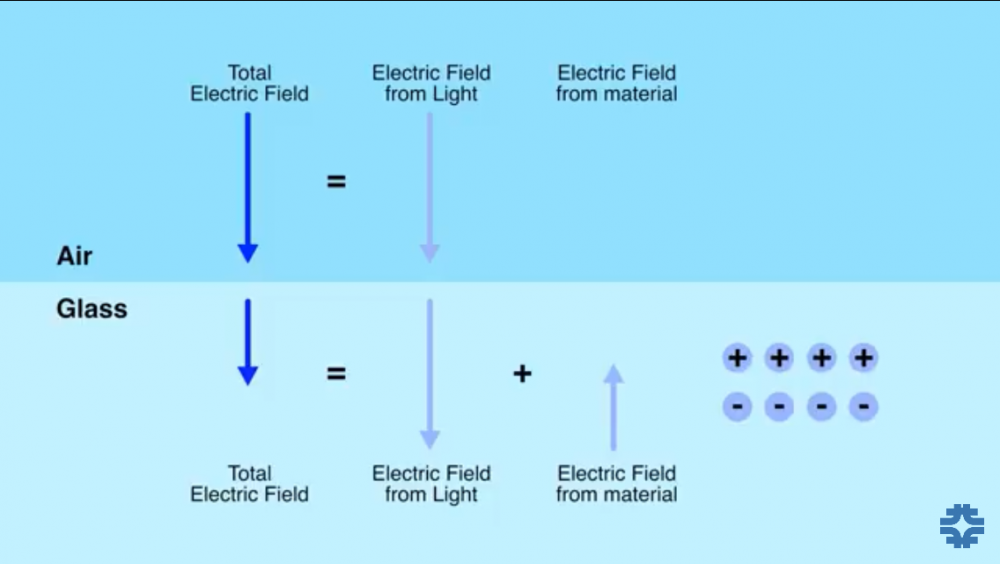
How do the charges in a glass set up their own counterbalancing electric field when an electric field from external source is imposed on glass?
in Classical Physics
Posted · Edited by King E
Typo
It was about epsilon, in case of refraction.