

cogujada
-
Posts
17 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by cogujada
-
-
Hey guys. Joigus and Studiot might remember me :)
I'm here because I have a question (not a doubt ;) )
Well, let me Introduce myself. I'm 18 years old, I study chem eng (first year, I'm a Freshman hahaha) in Madrid (so maybe my English is a bit weird, so, please, correct me in any errors you might observe). I've been having doubts about what to study since I was 15 years old. Last year (my last year in high school) I reduced my options to only two. Chemical Engineering and Chemistry. I tried to research as much as I could, but I lacked time, so I entered Chem (it was a very hard decision).
It's not that I don't like the Subjects, I love them. But I don't know if I'm going to like the work. I've been reading and I've seen that the knowledge of a chem eng about chemistry are very very poor (they know about Thermodynamics, Fluid Mechanics, Processes, etc). The work is repetitive, not very interesting... And i'm quite worried.
Firstly, I don't know if changing to Chemistry would be a good option (I wouldn't lose any year, because, at least in Spain, those subjects that you pass and are common to other subjects on other degrees don't need to be studied again). What really puts me back is that maybe I won't like as much the subjects (or maybe yes, who knows). I think the subjects in chem eng are much more practical (I mean practical not in laboratory, but in paper, there's much more about calculating, drawing, thinking, reasoning, etc. than in chemistry).
But if I stay at chem eng, maybe, I don't enjoy the career I develop, you know? I've seen that chemists have much more offers, much more enjoyable, non-repetitive works etc.
What do you guys think. Should I stay, or should I change?
You can ask for more information about my degree, what I like, etc.
Cheers!
0 -
Hey again.
Okay so first of all, I don't know if inversion is spanglish, but I have a question about DMO in TMO.
Inversion (at least in Spanish/Spanglish) is when the 2PI(bonding) orbitals have less energy than the 2SIGMA(bonding) orbitals, so they are completed with electrons before the 2SIGMA. I don't know if ' have explained myself, is is quite difficult to translate chemical terminology from English to Spanish.
This inversion occurs, for example, in B2, C2 and N2 (because the difference in energy between 2s and 2p orbitals is extremely low).
So hold your nose because here comes the question. Why CO suffers inversion and NO doesn't, I mean, why? Both of them have an atom that, by their own, "get inversed". I don't know if you understand me...
Thanks btw
0 -
2 hours ago, joigus said:
There's quite a bit wrong with Moeller's diagram. Some s/d orbitals (and some d/f I think) start violating it because they get very close in energy. If you try to fill in the levels by Moeller, you get the electronic configuration wrong. I think that's the reason why your teacher doesn't like it.
You can still use it if you remember the exceptions.
Yeah that makes sense to me. Some people told me that it was because Moeller's diagram didn't contemplate exceptions on Nb, Cr, Mo, Cu, Ag, Au, Pd and Pt electronic configuration, but I think (maybe I'm wrong) that Aufbau principle does not contemplate that either.
That makes a lot of sense to me. I was looking for some reason to stop using it, and I found one so in a way, you've changed my way of thinking, feel proud!
0 -
8 hours ago, studiot said:
This must be a very small part of your first year curriculum. I have never heard of Chemical Engineers requiring such detail
Well, in Spain we are told that "el saber no ocupa lugar". @joigus can translate it to you hahah
2 hours ago, joigus said:I don't think that's universal.
Yeah well I'm afraid I've been talking with other first year students (like me) but from other colleges (UAM, UB and UPM) and they don't even know about Schrödinger's equation. I think that she told us that to avoid messing our minds hahaha. I dunno really.
8 hours ago, studiot said:How are we doing?
Very very good. Thanks, I mean, you've both done more than any other person for me in my academic career I think

I know understand much better, not only Schrödinger's equation, but how difficult it gets chemistry when talking about insignificant electrons, what the hell man.
Thanks guys
2 hours ago, studiot said:My apologies to all.
hahahahahahah, mate you've been killing it out there explaining it all (along with joigus)... Hopefully, someday, my errors will be as insignificant as yours!
Best regards boys!
Just now, cogujada said:know
omg, now wtf is going on in my head
0 -
1 hour ago, joigus said:
Yes, for S (Z=16) and Cl (Z=17) the valence electrons are in 3p orbital, so those are outer electrons.
Yeah, and it stills follow the criterion of Higher Z, narrower graph and higher peaks (at least according to my teacher).
1 hour ago, joigus said:Yes, that's correct. Narrower --> Higher peaks. Conservation of probability.
Yeah, seems logical to me, but now that studiot posted the photo (thanks by the way), you can see that for higher Z, the graph is narrower but the peaks are lower (which seems non-sense).
1 hour ago, joigus said:I'm busy now. I'll get back to you in maybe 6+ hours...
No problem, my exam is the 15 of July, so I have still plenty of time haha
Thanks to both of you!
0 -
9 hours ago, cogujada said:
The red curve is R20, the dark green is R30, and the bright green (which you can barely see the humps) is R40
8 hours ago, joigus said:I agree with @studiot. Solving a high-Z atom is not like solving Schrödinger's equation for the hydrogen atom, substituting +e by +Ze and assuming that all the hydrogen-like "orbitals" are filled with electrons. There is the Hartree-Fock method, there are other methods that I can't remember now, and the problem is highly non-trivial.
You pointed to one of the clues yourself here:
It's even worse than that, AAMOF. You've got spin-spin effect, spin-orbit effects, the nucleus, London, and others I forget and would have to review. Highly complicated N-body problem mess.
But
intuition can guide you if you're interested in qualitative discussion. That's why I asked you if they were outer (valence) or inner electrons (1s). Outer electrons get more spread. But for very internal electrons my intuition (maybe my memory in part) tells me that it's the opposite. Electrons midway from both extremes are more difficult to predict.
I think it's no coincidence that they're giving you 1s electrons 1st principal quantum number and s-wave e-, so they don't stray very far from the nucleus-- for relatively high-Z atoms:
Those e- will be very close to a +Ze charged nucleus. External electrons, although many of them will be in high-l orbital angular momentum and stray farther from the nucleus, will, on the average, act as a shell of negative charge outside the internal ones. First approx. to this is a shell of negative charge covering them would be a spherical shell of negative charge which has zero electric field inside (I'm using electrostatics as a clue). It's not a sphere, I know, but we're thinking schematically. And AAMOF some of them are s-wave spheres!! So my intuition is 1s electrons will get closer to the nucleus (more "penetrating") in the case of the higher-Z neutral atom, even though the valence electrons suffer the opposite trend and spread out for higher Z.
Don't take for granted anything I say here. Take my cue, see if it makes sense, discuss it with your classmate, and do your research and finally do your own thinking. It's going to teach you a lot more than anything anybody can do by telling you the answer.
And plan B is ask your teacher. It may be embarrassing, but you must get over it.
 She won't mind, I'm sure. Quite the opposite. You can use my idea about internal electrons as a foil. I'm interested in the answer.
She won't mind, I'm sure. Quite the opposite. You can use my idea about internal electrons as a foil. I'm interested in the answer.
Edit: I can't see your picture.
yeah I'm afraid I need to ask her, however, what about the peaks? We've seen plotting the graphs (I think that if you click the hyperlink you can see it) that for higher Z, the graph is less spread out and has lower peaks, whereas our teacher told us that the peaks were higher (which makes sense, because the area under the graph has to be 1, and if you make it more narrow you'll have to make it higher no?).
On the other hand, I just saw an exercise about the 3p orbital of Cl and S, and it follows the same tendency (for higher Z, narrow graph and higher peaks). Do you consider them as outter electrons??
I want to know what you think about this before asking her
 0
0 -
12 hours ago, cogujada said:
Yeah well what I've deducted is that the radial wavefunction will be more spread out fore higher Z
Okay, so the guy that helped me out with this apparently got a bit confused and started to rethink what he told me (the radial wavefunction will be more spread out for higher Z). He has plotted this:
Where "The red curve is R20, the dark green is R30, and the bright green (which you can barely see the humps) is R40" As we can see, as Z gets higher, the first maximum gets nearer to the origin of the coordinates, and the peaks get lower. The first part (that the maximums are much closer to the y axis) seems good to me, however the second part (the peaks are lower) does not coincide with what my teacher told us. Do you have something to add @joigus and @studiot??
Thanks guys, you're saving my semester
0 -
24 minutes ago, studiot said:
But do not expect formulae they can only give trends
Don't understand this sorry hahaha
24 minutes ago, studiot said:Meanwhile read this recent thread about exceptions.
Extremely interesting, I knew Nitrogen and Oxygen were exceptions (and also P/S, Be/B and Mg/Al, but I didn't know about As and Se, I thought they weren't involved). I really want to thank you and @joigus, you're really talented guys and very helpful and kind.
26 minutes ago, studiot said:Sorry if my answer was too basic and simple.
No worries!!
Thanks very ver very much
0 -
8 hours ago, cogujada said:
Yeah well what I've deducted is that the radial wavefunction will be more spread out fore higher Z.
OMG, I don't know if this is correct, because I just seen another exercise made by my teacher which said that the radial wavefunction would be more spread out for the one with the lower Z. This is so frustrating
0 -
Hey, I study chemical engineering in Madrid (Spain).
Here, teachers do not like Moeller's diagram (my teacher, for example, has forbidden using it, and instead we use the Aufbau principle, which is similar). I don't know if this happens in your countries, but if it does happen, what are the reasons for this happening?
I mean, I don't think there's anything wrong with it.
Cheers.0 -
5 hours ago, joigus said:
That's certainly true for outer electrons. Are you sure it is also for inner electrons?
Well I don't know it for certain. All the exercises my teacher did were with outer electrons, except one, she did one with "inner electrons" (I at least consider 2s orbital as inner), and it was the same criterion, however, i'm not 100% sure.
4 hours ago, studiot said:Not sure why you need this for Chemical Engineering but good on you for wanting to know.
Well it's not that I want to know it (I do, I find really triggering chemistry) but our teacher thinks this is extremely important (Schrödinger's equation and the angular&radial part), so important questions about this topic were 50% of the exam.
4 hours ago, studiot said:Does this help?
Well, it is good to remember, but I already knew that, as I've said, this seems to be a very very important topic here in Spain (I have a friend who studies Pharmacy and she has been taught too the Schrödinger's equation). But it does not answer my question (I think).
I've been investigating and I've found that radial the wavefunction will be more spread out fore higher Z. @joigus helped me and told me that this is, without a shadow of doubt, true for outer electrons, but he's not sure about inner electrons.
You know, I could ask my teacher, but I have been asking her a lot of things (as you can imagine, virtual classes are by no means the same as "real" classes) and I'm a bit ashamed of asking her again (and in addition, I've already asked her about Schrödinger's equation, not this topic of polyelectronic atoms specifically, but a question about the angular part of an s orbital).
Thanks guys, you're great!
0 -
No it's not homework, it's something I found in the Internet. However I have a Chem exam (about this and more topics) in a few weeks.
Yeah well what I've deducted is that the radial wavefunction will be more spread out fore higher Z.
And thanks for correcting me!
Cheers
0 -
Hey, I study Chem. eng in Madrid (Spain), so first of all, my English might be awful, so I apologize.
I have a doubt about the radial part of polyelectronic atoms (you know, Schrödinger's equation etc). The radial part for Hydrogen is quite simple, but things get difficult when we talk about atoms with more than one electron (due to the shielding effect between electrons). My question is the following: How does the radial part of Schrödinger's equation change for polyelectronic atoms? I know there are a few ways of estimating the new radial part (SCF or STOs) but I don't need that. What I need is how does the graph change.
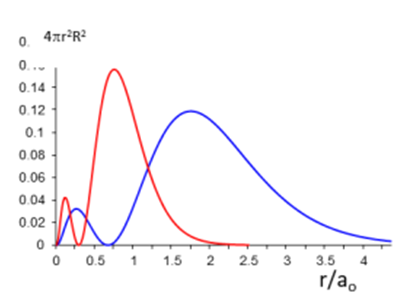
For example, here it is represented the 2s orbital of F and Li. How can I know which one is one in this case?
What really bothers me is that I want to know which factors influence the graph and if there is a general rule to know which atom is going to be more diffuse (in this case the blue line) or penetrated (the red line). For example, between two atoms, analyzing the same orbital (for example 2s and 2s, or 3p and 3p etc) the one with the highest Z is the most penetrated, and the one with the lowest Z is the most diffuse (obviously I don't know if this is true hahah, it is an example). You know, something like that so I can answer these type of questions:
Plot the graph of the radial part of S and Cl (1s orbital)
In order to answer this, what I need is to know which one of them is more penetrated.
I don't know if you understand me. Again, sorry, my English is awful...
Thank you guys, if there's something you don't understand please tell me and I will answer shortly. By the way, feel free to correct me in any grammatical, spelling error please, I really want to boost my level of English!
Cheers
0 -
Really don't know what to say hahaha. Thanks to you all guys, I will try to improve my English everyday

And for those who might curious, i'm gonna buy the book, 31 pounds is not money at all when we are talking about knowledge. Thanks!
0 -
11 hours ago, studiot said:
No single book will be the 'most advanced' in any technical subject these days.
Also since you are going to University or Polytechnique you will be taught things on your course that are not in any book.
I remember one Professor coming in and saying "You will not find this reaction in any book - I only discovered it last week.)About the Housecroft book
It is already far more than you will probably ever need 1285 pages in the 2018 edition.
As you say you are studying Chem Eng, not Chemistry.
My flatmate at Loughborogh studied Chem Eng and didn't have any heavy Chemistry books.
Is it the recommended course book?
In which case there will probably be exercises from it that you will need.
Robert Gordon University Pharmacy publishes a list of recommended books and articles and notes whether ther are just for looking at, extra reading or 'essential'.
Find out if your institution does this and play close attention to it>
Do not miss any reference they call essential - it may come up in the exam.There will be plenty of stuff (and higher mathematics) in Chemical Engineering that Chemists do not study.
Put your best efforts into that would be my advice.Yeah well, you've got a very interesting point. As a chem eng probably I will use the book only for specific consultations (except this year, I have a subject called Inorganic Chemistry). However, bare in mind that I've found the book at a really cheap and good price, usually, the Spanish version costs 95 € (85 pounds) but I've found a second-hand one for 35 € (31 pounds) which is a third of the original price. Do you think that it is profitable to buy the book (taking into account that I will only use it for specific consultations or to know more about inorganic chemistry)???
Thanks mate!
And sorry, I forgot. It is the recommended coursebook. Sorry haha
0 -
Hey, I study Chemichal engeneering in Madrid (Spain) and I have a doubt about a book. Do you know if the book Inorganic Chemistry (written by Catherine Housecroft) is the most "advanced" book about Inorganic chemistry (I mean, can I found books much more advanced and more profound, or is the Housecroft valid). I mean, im not going to work as a pure chemist (I think), but I dont want to spend loads of money if I can find better, profound and advanced books (considering that I study Chemical Engeneering and not pure chemistry, obviously).
Thanks guys, and sorry for my English, tell me if there's something you dont understand!
Cheers0

Chem Engineering or Chemistry?
in Applied Chemistry
Posted
Alright guys so I finally decided to stay in Chem eng, due to the fact that I love the subjects and I don't really know if i'm going to like pure chemistry as much. I don't know what Bs C stands for, but just in case, I was doubting between changing from the chem eng degree to the chemistry degree (at the same University, by the way).
I think that choosing the degree for the work you're gonna do is not very intelligent. I mean, jobs change a lot during the curse of the years. I'll stay as I am hahaha
Thanks guys!!!