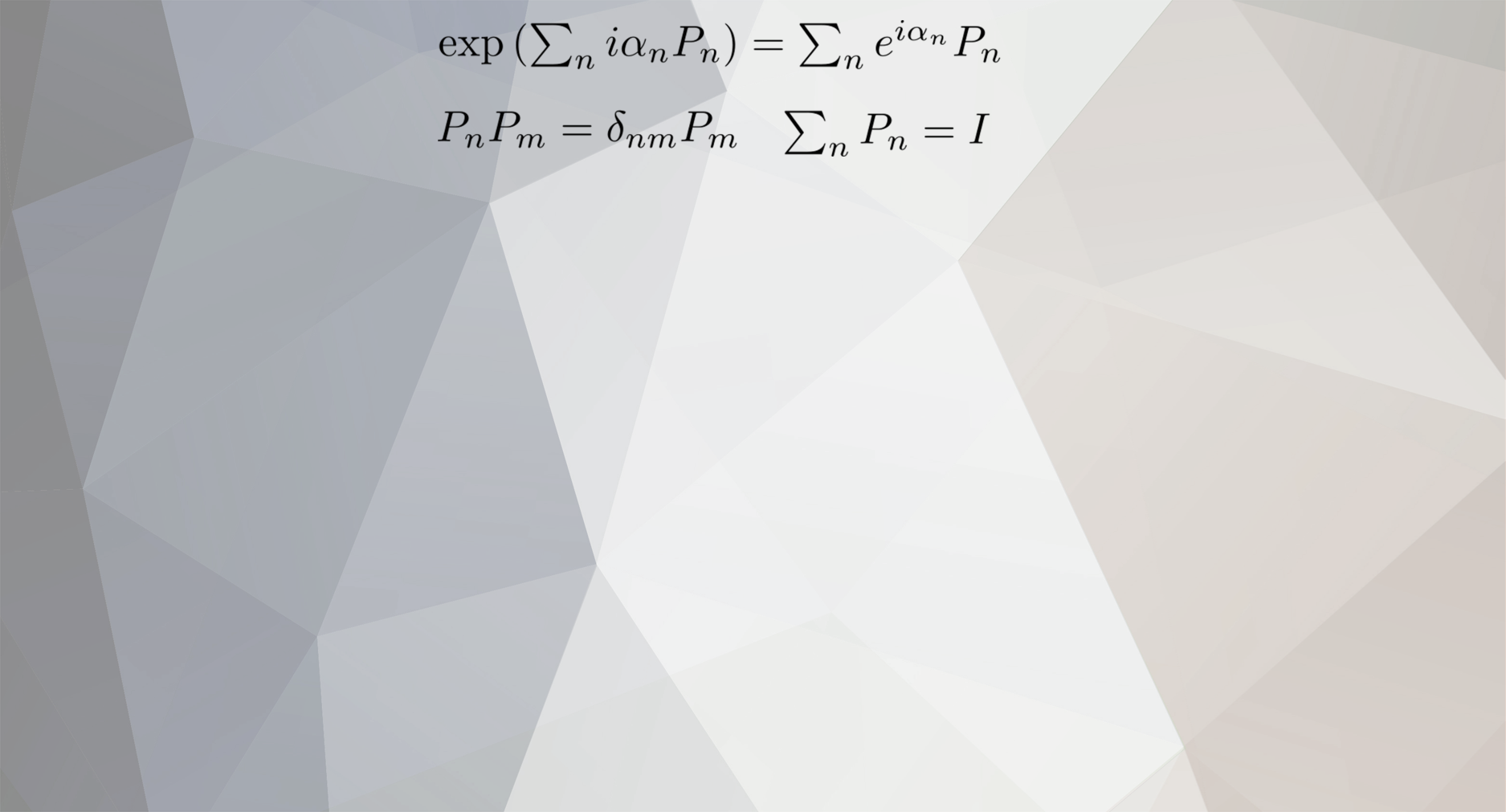-
Posts
4386 -
Joined
-
Days Won
49
joigus last won the day on March 5
joigus had the most liked content!
About joigus
- Birthday 05/04/1965
Profile Information
-
Location
(0,0,0)
-
Interests
Biology, Chemistry, Physics
-
College Major/Degree
Physics
-
Favorite Area of Science
Theoretical Physics
-
Biography
I was born, then I started learning. I'm still learning.
-
Occupation
teacher
Recent Profile Visitors
27164 profile views
joigus's Achievements

Scientist (10/13)
1k
Reputation
-
What do you mean non-relativistic photons? Photons are always relativistic. Am I missing something? Everybody else seems to understand what you mean, but I don't. Because they're always relativistic, the equation of state (pressure as a function of density) is what it is in the matter term of the Friedmann equation.
-
It seems like they do. Close to 50% as much carbon as fluorine for fluoroalkanes. More if double bonds C=C or higher occurs (or other radicals). Linear chains 2n+2 F's per n C's cyclic chains 2n F's per n C's 2-cyclic chains 2n-2 F's per n C's That assuming nothing else is goin on but C-F bonding, which sounds pretty boring. Carbon is still the structural scaffolding in FC's. And F is monovalent, which leaves little room for anything interesting going on IMO.
-

Determinism shown to end contradiction
joigus replied to jnana's topic in Modern and Theoretical Physics
This definition of the principle of causality as applied to classical mechanics is indeed a meaningless nonsense one, as you don't include time in it, or the paradigm of the differential equation, which is the way in which causality is implemented in physics. Fortunately neither Newton, nor Laplace, etc said anything of the kind. It is, rather, state of A, B, C at time t1 causes state of A, B, C at time t2, which causes state of A, B, C at time t3, and so on. -
Molecules can vibrate, rotate, twist, and scissor, etc. In fact, there are DOF that are not obviously rotational/vibrational, etc. but some complicated so-called normal modes like, https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Vibrational_Spectroscopy/Vibrational_Modes/Number_of_Vibrational_Modes_in_a_Molecule Recently, a key to why CO2 (kinda mysterious, as it's just a boring non-polar linear molecule) is such an important agent in global warming has been found to have a root in resonances of such non-obvious normal modes due to Fermi resonances: https://arxiv.org/pdf/2401.15177.pdf
-
Sorry. Here's a lowdown of the vocabulary I've used. Tell me, please, where the problem is: Degree of freedom Temperature Specific heat internal (rotational/vibrational) vs external (CoM) cutoff (making some energy --or wavelength-- domain irrelevant; see next) freezing (as in freezing degrees of freedom by making them very unlikely to store energy under thermal-equilibrium conditions).
-
Absolutely. Sorry I missed this very good argument for so long. It's only because of what you say that different molecules have different specific heats as a function of temperature. The internal degrees of freedom are totally relevant. This is exactly the reason why different molecular components have different specific heats. What other reason could there be for different gases to display different specific heats if only the CoM DOF were relevant? Quite a different matter is how quantum mechanics introduces a cutoff for short-length degrees of freedom (independently of how poly-atomic a gas is), and how this played a crucial role in the dawning of quantum mechanics itself. (Birth of the old quantum theory as a mechanism to freeze the short-wavelength DOF.)
-
Agreed. @martillo's suggestion is not going to work. Your suggestion is, if I understand correctly, a valiant attempt --let's put it that way-- to try and make sense of their hopeless intention to define temperature as an attribute of one molecule or atom. I think you're right to say that the ergodic theorem is essential to define thermodynamic equilibrium. If most typical physical systems we deal with were not ergodic, I don't think statistical ensembles would work at all within the context of variables such as the partition function, temperature, Helmholtz's free energy, entropy, and such. It would be a disaster. The least I can say is those variables would be as good as useless. So why bother trying to make sense of something that just doesn't? Just to spite me? Temperature is an ensemble-related parameter. There is no operational definition that would allow us to measure the temperature of a molecule either. There is no theoretical framework that allows us to define it in such a way except by way of the ensemble. Temperature is an ensemble property. Even more so than entropy is. At least the microscopic entropy of a molecule can be defined as the volume of phase space for that molecule, which is always the same. Not so for temperature.
-

Bohmian Locality as an answer to Bell's inequalities
joigus replied to JosephStang's topic in Speculations
Sorry, pseudoscience is beyond my scope. I'm out. -

Bohmian Locality as an answer to Bell's inequalities
joigus replied to JosephStang's topic in Speculations
I'm sorry I was disrespectful to the geometry[?!]. I think you're trying to talk about the Arahonov-Bohm effect. Now I'm positive that's how you connected the words "Bohm" and "holonomy". It's about this: https://en.wikipedia.org/wiki/Aharonov–Bohm_effect Aharonov-Bohm holonomy has nothing to do with realism, locality, or any of that, even though the word "Bohm" appears there too. It's the De Broglie-Bohm theory that does. Kinda like the Maxwell-Boltzmann distribution has very little to do with Maxwell's equations, except for Maxwell. Do these comments help a little bit? -
Hey, nice account. BTW, I recommend you Copenhagen, by Michael Frayn. It's about that (in)famous meeting, and offers a possible development that I can only conceive as happening with a many-world view.
-

do you believe demon possessions and fallen angels are real
joigus replied to knowledgeispower917's topic in Religion
From what I know (and I know one case personally) it wasn't obvious during pre-pubescent stage and there were environmental factors that triggered it post-puberty. So what you say checks with my personal experience. That's why gene-based diagnosis is sure to become essential in the future. -
Only when a system is in thermal equilibrium, and provided it is ergodic, the time average of the kinetic energy coincides with the ensemble average at any one time. So you need the ensemble plus the fact that the system be in thermal equilibrium plus ergodicity. Very very special conditions indeed. And when it works, you've used the whole ensemble to define it... So it's not a property of the particle. It's a property of the ensemble! So no --I insist--, there is no temperature as a property of a singled-out particle of an ensemble in general. And when there is such a thing, it's only by stretching the concept so that what really is a property of the ensemble is decreed to be a property of any and every one of the members of the ensemble. I don't see how this definition does anything, really. And believe me, I would like nothing more than to be illuminated about anything physics.