-
Posts
8 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Pharmacist
-
-
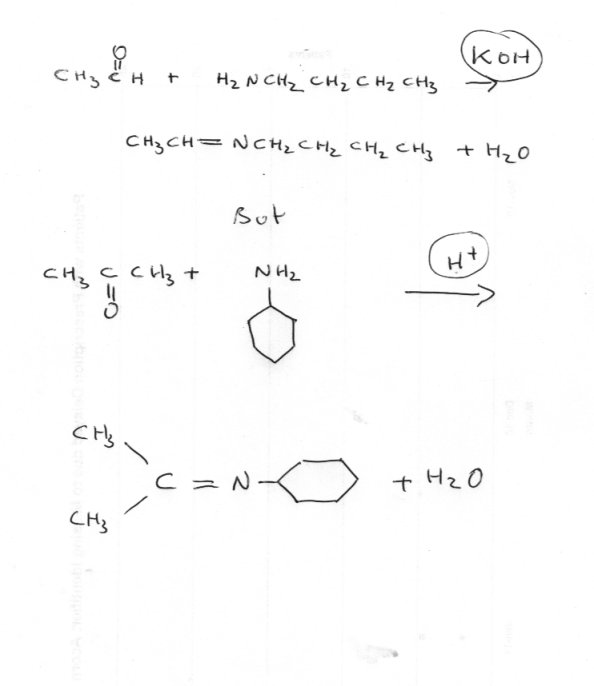
Hello
I am confused most papers says schiff base complexation with metals resulting in lower shift in Ir spectrum of azomethine group.As far as I know decreasing e density leads to increase the frequency vibration.However after intense inspections, I found two papers says it causes to increase the freq.Is it possible for both??0 -
Good day everyone,
I aimed to synthesize schiff base complexe with CuCl2. 2H2O.
But I only have an aldhyde Ir spectrum from which its schiff base was made?!!!
Why this is happened?
The reaction was as follow :
I added cu metal unhydrous ethanolic sol. Dropwise to hot ethanolic sol. of schiff base then reflex for about 3 hrs.
0 -
Good day everyone,
I wonder if schiff base is stable on TLC plates of aluminum oxide?
I know it is hydrolysed on silca gel.
How about aliminium oxide?
0 -
5 hours ago, studiot said:
My reactants are dioxane, CH2Cl2 and CHCl3 soluble.
I have tried dioxane and ethanolwith different ratios
Also I have tried ethanol and minimal CHCl3.
On TLC all reactant appeared and no obvious new spot.
I think my low yield schiff base is being hydrolysed on TLC "aliminium oxide"
I will try CH2CL2 WITH Mgso4 and glacial a.a
If not succeed, I will try dean stark trap with toluene although the reactants aren't soluble in it.
My reactants are dioxane, CH2Cl2 and CHCl3 soluble.
I have tried dioxane and ethanolwith different ratios
Also I have tried ethanol and minimal CHCl3.
On TLC all reactant appeared and no obvious new spot.
I think my schiff base is being hydrolysed on TLC "aliminium oxide"
I will try CH2CL2 WITH Mgso4 and glacial a.a
If not succeed, I will try dean stark trap with toluene although the reactants aren't soluble in it.
0 -
Good day,
I hope you answer my question.
1.
I am trying to synthsis schiff base, should I dissolve my reactants?
Because they are not totally be dissolved even in hot solvent" ethanol"
0




Esterfication of carboxylic acid with alkyl halide
in Organic Chemistry
Posted