

danking
-
Posts
37 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by danking
-
-
26 minutes ago, swansont said:
Phi for All, who posted the modnote.
omg.... is this what the evil one has cum too?
seriously
 -2
-2 -
34 minutes ago, hypervalent_iodine said:
Er, what? If the electron thing was a red herring then we are once again back to the fact that your sequence is wrong, with no explanation as to why this doesn't matter. Your whole idea relies on this sequence and it's similarity to another sequence. It has been shown to you in detail how and where it is not similar, and rather than admit that maybe you were wrong (there's no shame in that), you instead try to change physics to make it similar. When this is pointed out, suddenly f orbitals don't exist, or it's a red herring. Can you see how this might come across ridiculous?
Back to orbitals. It is clear that you do not know what they are, or even the history of how we came to our current understanding. I encourage you to change this and go read up on them. Until you can understand the mathematics and physics that allow us to construct the orbitals we all know and love today, you are in no position to tell anyone that it's wrong (much less propose your own idea).
Minor correction. I am a PhD student, I don't tutor them. I tutor chemistry all the way up to third year level.
danking, I have not given you any rep points, positive or negative. That's not to say your posts haven't warranted them, though.
Rest assured, you are not Galileo.
How do you know Mr Iodine?
3 minutes ago, swansont said:I deleted my own post after I saw that Phi had already replied. You, too, have the ability to edit what you post (for a limited time)
Why should I who is Phi?
It's like repent... sinner...
0 -
"No, we don't delete threads, unless they are in violation of the rules (i.e. spam or profanity)"
OMG apparently that is not true:
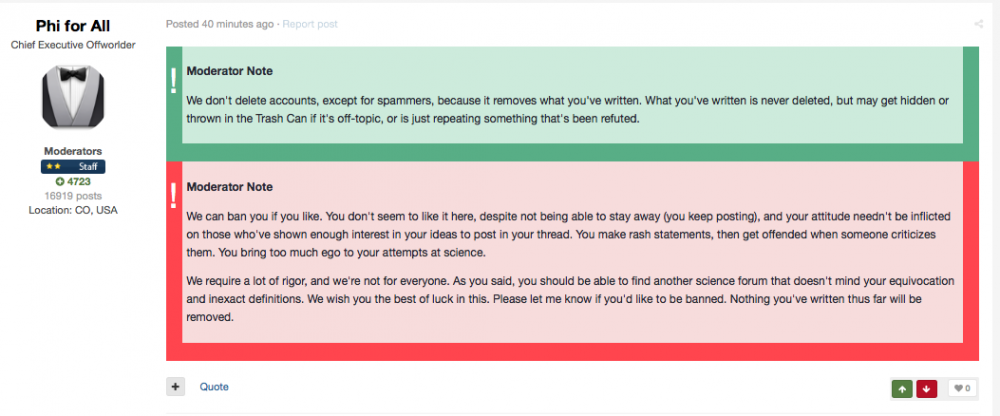
very Chinese of you... lol ... who needs Free Speech lol!
2 hours ago, studiot said:77 replies and 725 views of this subject........... about 10 views per reply.
No I don't believe that either swansont or HypervalentIodine have downvoted you, that that is obviously possible.
and by the way, yes you are new, and if you had taken the time to look around you might have noticed that HypervalentIodine is in fact a Lady who tutors Phd chemistry students.Lots of members have passed through, look and say to themselves
"Do I want to deal with this poster"
You should reflect upon the answer to that.
I have already told you why I have continued to bother with this thread.
You have not picked up on that or asked anything about this.
It is a fact that many apparently unconnected physical phenomenon appear in abstract mathematical group theory.
I have retired and am not interested in further glory, as I have a dozen or more scientific papers to my name. If I have prompted you in that direction and it bears fruit, you are welcome. It will have advanced science.
But note that this thread has made its way to the trash can for other reasons.No, we don't delete threads, unless they are in violation of the rules (i.e. spam or profanity)
This was deleted FYI...
#
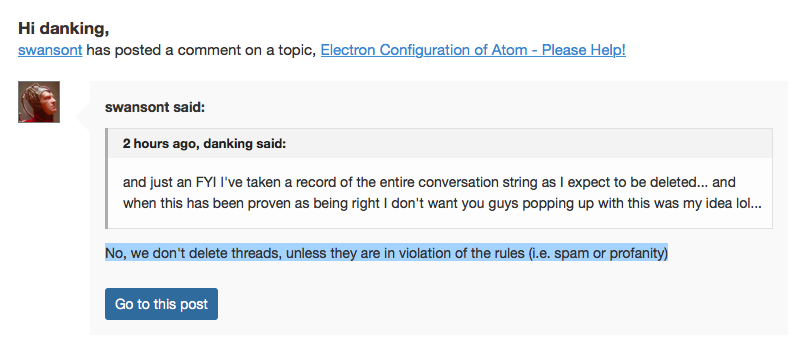
which when you click on Go To this post
speaks for itself `I guess...
Look I'm not interested in shining a light on your hypocrisy but you keep emailing me:
and yet I have said don't...
so this is your bad UX not me...
Pretty MUCH like my experience here... bullying...
poor UX - user experience ...
poor...
pleasssseeee stop emailing me i am soooooo over this lol
- This is a great gem considering i am a reasonable human being very North Korea lol
Should you delete your forum maybe?
-1 -
God Save the Tash Can...
Pllllleeeeeaaaassssseeee delete me...
0 -
1 minute ago, studiot said:
You are just reacting you are not thinking and you are certainly not reading what is written.
I have no idea and no way of finding out who is downvoting you.
It has been at least two years since I last gave a negative vote, I try to only use positve ones for encouragement.And I have a personal policy of stating the reason in my next post any time I vote up or down for anybody.
I have never been ashamed of my opinion (which is what that is).But I have already told you that I have not voted in this thread.
No I was not referring to swansont.
Okay well look I have 3 down votes... I'm sure galileo had more than 3 down grades... i expect it as a complex thinker it's a concrete thinker reaction to a complex thinker...
There have been only 4 main replies so I don't have to be Agatha Christie to work out the downgrades...
As a new user can I tell you how you come across?
You come across as bullies... you use passive aggressive statements and then accuse people of being emotional... it's pretty tragic and I guess when you can't bully them you downgrade them in frustration.. pretty tragic for a science forum that is supposed to be free from emotion.
You accuse people of behaviour you exhibit.
I wish you, guys well but my guess is that you are the only people that post because your users are scared of you... your default setting is to attack, accuse and humiliate... that's neither human nor scientific.
sorry lol not swansot lol: time bandits evil genius
Evil defo...
and just an FYI I've taken a record of the entire conversation string as I expect to be deleted... and when this has been proven as being right I don't want you guys popping up with this was my idea lol...
Whose evil now... lol
-1 -
2 minutes ago, studiot said:
Yes you have answered some of my questions clearly, but wrongly.
Would you like a list?
Within 5 minutes of my post you reproduced as fact that which another member with an impeccable academic pedigree has told you was 'incorrect'.
Why?
3yes please... list...
I'm sorry but I thought science wasn't personal? Don't get "emotional"
What does this mean???
Within 5 minutes of my post you reproduced as fact that which another member with an impeccable academic pedigree has told you was 'incorrect'.
who is this member? OMG seriously you are s SCIENCE TROLL...
What this guy with the hat?
Your serious right? The trajectories?
Can I assume if I guess you, Mr Swansont with the "impeccable academic record" and Mr Iodine and given me a bad sign on my profile lol...
You like your own opinions and nobodies else... very sad... there are tons of science forums ... i will go elsewhere... ;-) #holdnogrudge
this is your space...
can't you just delete my account? the silence of the lambs an all that...
Flat earth?
4 hours ago, danking said:It's omitted because there is no gap between 1 2 3 gaps = 0 0 so you could write 0 0 1 1 3 1 3 1 3 5 1 5
I've also had a chance to drill into this p and d problem and have an explanation...
Summary of the main issues yesterday was the p and d are in the wrong order below... should read 5 3 not 3 5 (I've not looked at f because that is a lot more full on)
1 1 3 1 3 1 3 5 1 5 (Primes Gaps)
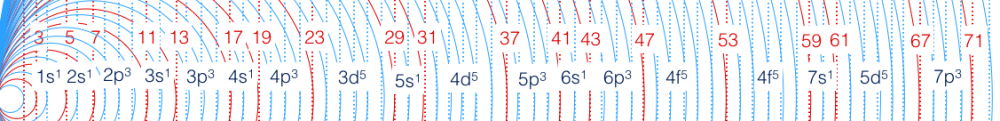
1s1 2s1 2p3 3s1 3p3 4s1 4p3 3d5 5s1 4d5 (Electron Configuration from Hydrogen to Cadmium)
Okay so the 4p and 3d are in the wrong order... why?
What is the difference between p and d?
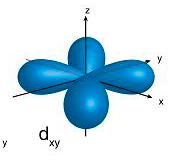
P orbital
d oribital
There is a significant difference...
p orbitals contain 2 electrons on each axis x, y and z
d orbitals contain ONLY 1 electron on each axis AND occupy the same space as the px, py, pz, s = 4 electrons
and in between the x, y and z 6 electrons
So essentially the electrons in the d occupy 10 axis NOT 5... and are in the "same space" as the p and s BUT they go through the middle of the nucleus as there is only 1 electron per axis...
So the d orbitals occupy:
4s1, 4p3, 3d5, 5s1 BUT across the axis so they also occupy the - 4s1, - 4p3, -3d5, -5s1 with the SAME electron... = 10 electrons
I haven't looked at the f orbitals I thought I would let this sink in first - so please don't bombard me with f lol
Dan
2 hours ago, danking said:Okay good we're getting somewhere you are absolutely right the electron aspect is a bit of a red herring because they are probability shells rather than actual paths ways themselves ... The key is they sit within those "shapes" so what is keeping them in those shapes? If you add electrons together in the same space you get sparks or lightning...
The prime numbers are the key... they make those "cone" shapes...
It's very hard to show in 2D but this is a prime number "cone"
okay this is a bit of a nonsense ... the 0 atom? 1 2 3 could very well be the dimensional aspects i.e. the axis and whether 1 or 2 are primes is a nonsense...
and if you read the last post those are single spin atom allocations - d block so the existence of a single spin in d would translate to other single spins but this is all a bit like 100 and 1,000's on a cake... you need the cake first.
More complex version of the prime cone... (red lines indicate primes)
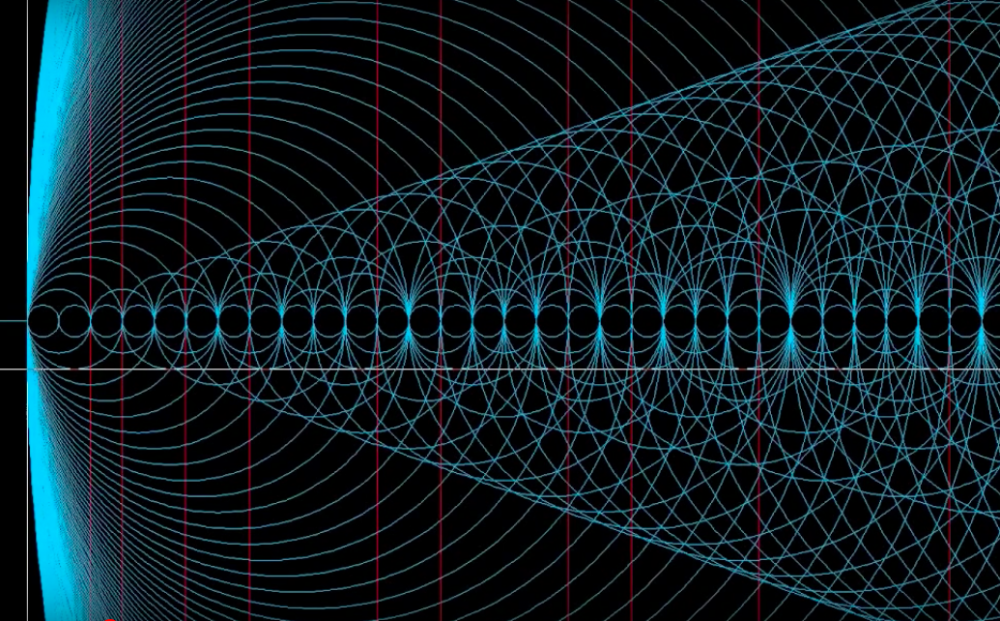
and more complex sitll
you can see with a little imagination the cones within each other... on a particular axis
0 -
Okay so the tone on here was initially against me...
Tonally it is very combative.
and when you are wrong you simply accuse me of being very rude... I have been accused of being stupid etc which is fine but it is a little much - Even recently I was attacked as not "knowing x,yz" it's VERY passive aggressive.
I am a mirror of how I am being treated so if you see me as rude... that is how you come across.
On 28/08/2017 at 2:21 PM, studiot said:Instead of thanking me every post and then totally ignoring what you are allegedly thanking me for, please pay attention to what I am saying and address my points.
I hope they are quite simply put.
And I do address the points you make.
This was you... stop thanking me... "please pay attention"
On 28/08/2017 at 9:22 PM, swansont said:It's unfortunate if you think this is personal. That's not something that ends well in science. Most ideas are wrong. Being emtionally invested in them is a bad idea.
Honest evaluation is what you sign up for on a science discussion site.
Again another "personal quote"
etc etc... there are plenty of examples and it got worse - it feels like you have run out of answers so now you will delete me or "down play" the post regardless...
BUT i would thank you all because it has progressed the idea... and it is a shame if I need to go to a new forum to discuss this new concept... because needless to say if it is right your forum would be famous
 13 minutes ago, studiot said:
13 minutes ago, studiot said:Yes you are being rude, very rude.
This thread is littered with fallacious statements by you.
Each time one is pointed out by someone ( not all by me ) you carry on as though they had not posted.
Perhaps that is why others (again not me) have been downvoting you.
I am only pursuing this now because the partial match between the two sequences is interesting and I wonder how far it can be taken or if there is anything in modern abstract maths that would apply here (perhaps group theory).
You asked for help yet sem unable to accept any or conduct a rational discussion progressing to a better conclusion that the initial hypothesis.
2You are distracting from the science... this is on the homepage?
I've answered your questions... clearly.. you normally bombard people but that has stopped so you are either bored or can't come up with an "explanation" from Google...
Yesterday was like a free for all.. and now I am accused of being rude lol Pot Kettle Colour Check Table 5 ;-)
and it's not that downplayed?
 1 hour ago, danking said:
1 hour ago, danking said:Okay good we're getting somewhere you are absolutely right the electron aspect is a bit of a red herring because they are probability shells rather than actual paths ways themselves ... The key is they sit within those "shapes" so what is keeping them in those shapes? If you add electrons together in the same space you get sparks or lightning...
The prime numbers are the key... they make those "cone" shapes...
It's very hard to show in 2D but this is a prime number "cone"
okay this is a bit of a nonsense ... the 0 atom? 1 2 3 could very well be the dimensional aspects i.e. the axis and whether 1 or 2 are primes is a nonsense...
and if you read the last post those are single spin atom allocations - d block so the existence of a single spin in d would translate to other single spins but this is all a bit like 100 and 1,000's on a cake... you need the cake first.
More complex version of the prime cone... (red lines indicate primes)

and more complex sitll
you can see with a little imagination the cones within each other... on a particular axis
2Back to the science lol...
3 hours ago, danking said:It's omitted because there is no gap between 1 2 3 gaps = 0 0 so you could write 0 0 1 1 3 1 3 1 3 5 1 5
I've also had a chance to drill into this p and d problem and have an explanation...
Summary of the main issues yesterday was the p and d are in the wrong order below... should read 5 3 not 3 5 (I've not looked at f because that is a lot more full on)
1 1 3 1 3 1 3 5 1 5 (Primes Gaps)
1s1 2s1 2p3 3s1 3p3 4s1 4p3 3d5 5s1 4d5 (Electron Configuration from Hydrogen to Cadmium)
Okay so the 4p and 3d are in the wrong order... why?
What is the difference between p and d?
P orbital
d oribital
There is a significant difference...
p orbitals contain 2 electrons on each axis x, y and z
d orbitals contain ONLY 1 electron on each axis AND occupy the same space as the px, py, pz, s = 4 electrons
and in between the x, y and z 6 electrons
So essentially the electrons in the d occupy 10 axis NOT 5... and are in the "same space" as the p and s BUT they go through the middle of the nucleus as there is only 1 electron per axis...
So the d orbitals occupy:
4s1, 4p3, 3d5, 5s1 BUT across the axis so they also occupy the - 4s1, - 4p3, -3d5, -5s1 with the SAME electron... = 10 electrons
I haven't looked at the f orbitals I thought I would let this sink in first - so please don't bombard me with f lol
Dan
it has been really useful so thank you all - sorry it got heated at times BUT collaboration is never easy!
0 -
You are continuing to avoid the question.
When you have paired electrons you can guarantee one of each spin so you can choose a set of all one spin.
But if you have a single extra electron it could have either spin and you cannot know which.
Last time you avoided the question by telling me that it does not matter because the spins are the same.
No I answered this a while ago but lets explore...
Okay so here is an electron...
what do you think their spin is?
One has an anti clockwise spin and is travelling UP
The other has a clockwise spin and is travelling DOWN
Agree?
5 minutes ago, studiot said:37 minutes ago, danking said:okay this is a bit of a nonsense ... the 0 atom? 1 2 3 could very well be the dimensional aspects i.e. the axis and whether 1 or 2 are primes is a nonsense...
So you are saying you really don't know what a prime is then if you don't know if 1 or are prime numbers.
Here is a page full of explanations as to why 1 is defined to be not a prime number, but 2 is.
But what matters is that the first number in the prime gap sequence is 0
But the first number in the electron sequence must be a 1
OMG you need to lay off Google lol...
I'm not being rude but this is nonsense... I know you got the electron configuration wrong originally but please lets not over do it with Wiki links...
I'm cool with Critical Thinking but not with "critics"
and I showed you yesterday that spin is perspective based so it's nonsense i.e clockwise and anti-clockwise are they same thing...
just look at a clock take it off the wall and look it from behind it goes the other way... so your right UNTIL you look at it you don't know which way it is going...
QuoteYou are continuing to avoid the question.
When you have paired electrons you can guarantee one of each spin so you can choose a set of all one spin.
But if you have a single extra electron it could have either spin and you cannot know which.
Last time you avoided the question by telling me that it does not matter because the spins are the same.
 and you can try this at home on a sheet of paper...
and you can try this at home on a sheet of paper...
also Google water going down a plug hole in Australia... opposite to here
PERSPECTIVE ... it's the same way they are just seeing it from the other side...
 -1
-1 -
1 hour ago, hypervalent_iodine said:
Your explanation for the differences in p and d orbitals further leads me to believe you don't actually know what orbitals are. They aren't static orbital pathways for electrons. They are probability density maps. Those shapes are just a representation of the space in which you are likely to find a particular electron.
Okay good we're getting somewhere you are absolutely right the electron aspect is a bit of a red herring because they are probability shells rather than actual paths ways themselves ... The key is they sit within those "shapes" so what is keeping them in those shapes? If you add electrons together in the same space you get sparks or lightning...
The prime numbers are the key... they make those "cone" shapes...
It's very hard to show in 2D but this is a prime number "cone"
29 minutes ago, studiot said:1 is not a prime number.
So there is only one zero in the prime gap sequence.
That does not explain why you feel entitled to omit it. Zero is a valid number.
So your sequence does not match at the very beginning (there are no atoms with zero electrons).
You still have not answered my question about arbitrary spin allocation to atoms with odd numbers of electrons
okay this is a bit of a nonsense ... the 0 atom? 1 2 3 could very well be the dimensional aspects i.e. the axis and whether 1 or 2 are primes is a nonsense...
and if you read the last post those are single spin atom allocations - d block so the existence of a single spin in d would translate to other single spins but this is all a bit like 100 and 1,000's on a cake... you need the cake first.
More complex version of the prime cone... (red lines indicate primes)

and more complex sitll
you can see with a little imagination the cones within each other... on a particular axis
-1 -
14 hours ago, studiot said:
As a furthr matter of interest,
Why did you omit the first prime?
This would have started your gap sequence with a zero.
It's omitted because there is no gap between 1 2 3 gaps = 0 0 so you could write 0 0 1 1 3 1 3 1 3 5 1 5
I've also had a chance to drill into this p and d problem and have an explanation...
Summary of the main issues yesterday was the p and d are in the wrong order below... should read 5 3 not 3 5 (I've not looked at f because that is a lot more full on)
1 1 3 1 3 1 3 5 1 5 (Primes Gaps)
1s1 2s1 2p3 3s1 3p3 4s1 4p3 3d5 5s1 4d5 (Electron Configuration from Hydrogen to Cadmium)
Okay so the 4p and 3d are in the wrong order... why?
What is the difference between p and d?
P orbital
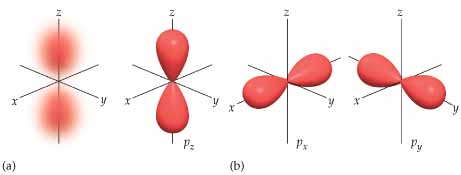
d oribital
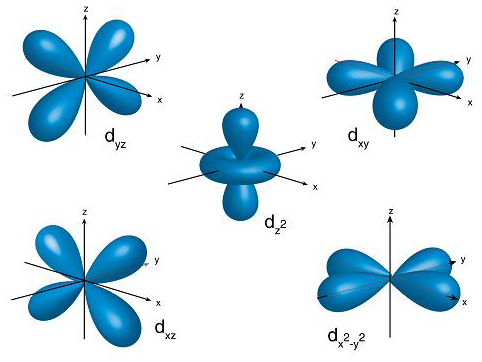
There is a significant difference...
p orbitals contain 2 electrons on each axis x, y and z
d orbitals contain ONLY 1 electron on each axis AND occupy the same space as the px, py, pz, s = 4 electrons
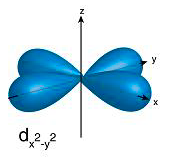
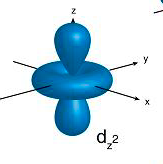
and in between the x, y and z 6 electrons
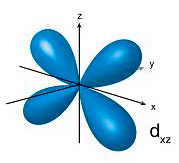
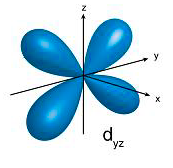
So essentially the electrons in the d occupy 10 axis NOT 5... and are in the "same space" as the p and s BUT they go through the middle of the nucleus as there is only 1 electron per axis...
So the d orbitals occupy:
4s1, 4p3, 3d5, 5s1 BUT across the axis so they also occupy the - 4s1, - 4p3, -3d5, -5s1 with the SAME electron... = 10 electrons
I haven't looked at the f orbitals I thought I would let this sink in first - so please don't bombard me with f lol
Dan
0 -
Yes sorry I couldn't resist... no monatomic doesn't mean with 1 electron it's either with 1 extra or without one:
ono atomic hydrogen is neither hydride (H-) nor proton (H+). Rather, what you seek is the H radical. Typically, hydrogen free radicals are so reactive that they don't exist in solution but their chemistry can be accessed by reacting longer lived radicals with hydrogen atom donors in a reaction called a hydrogen atom abstraction. Alternatively, it is possible to generate hydrogen atoms in solution via photolysis or high temperature in molecules with particularly weak R-H bonds. In these cases the H radicals will react very rapidly and are typically very short lived.
Cheers
There is no odd or even or clockwise or anti-clockwise its perspective.
Sorry that should say mono atomic ...
0 -
Yes so in terms of spin... or clockwise and anticlockwise.
If you look at your clock it is going clockwise I assume so you can tell the time?
If you go behind your clock and look at it from behind it is going anti clockwise...
You can see this on a piece of paper with a thick pen
This circle is clearly spinning clockwise in the + x axis
But if I am on the other side of the page it's going the opposite way:
If you watch cyclist bike wheels from one side of the road then the other you can see the same thing...
So spin is relative.
In terms of atoms and elements... Li is an element with a single electron BUT it doesn't exist by itself it reacts very quickly... Oxygen "free radicals" are the same and age us by destroying the DNA very reactive... it's the reason chemistry happens.
If you want to read up on orbitals you can see the only difference between the p and d is their axis of orientation
They're the same shape they just sit in different axis so all this argument around 4p and 3d is an axial argument i.e is the shell sitting in the x or xy axis? i.e. the p or d... and f is LOTS more involved...
0 -
Hi
5 minutes ago, studiot said:Here is a prime (smile) example of why we should have a numbering or indexing system on the posts like previous versions of this forum used to.
danking you are having trouble quoting. (me too)
One thing I have discovered with this new improved presentation of the forum is that if you highlight (select) a portion of text and right click, then a black 'quote this' balloon appears.
Click on that and you get the quote as above.
I wanted that quote because whilst playing with a spreadsheet this afternoon to compare sequences I realised the following problem with your derivation of your sequence.
You have divided the electron population in half to obtain the up spin electrons for entry into your sequence.
But
This will only work for paired electrons.
Every unpaired electron will be automatically undecided as it could be up or down.
You cannot arbitrarily chose it to match the spin of the rest of your electrons.Every other element in the table will have an unpaired electron, and some will have more.
yes this forum needs a little something to make it easier... the post trail has gotten very long.
the spin thing is really interesting...
firstly the elements don't exist as elements - atoms unless they are noble gases i.e. single electrons don't exist...
but in terms of the spin, it's either up or down or clockwise or anticlockwise
so they look like this:
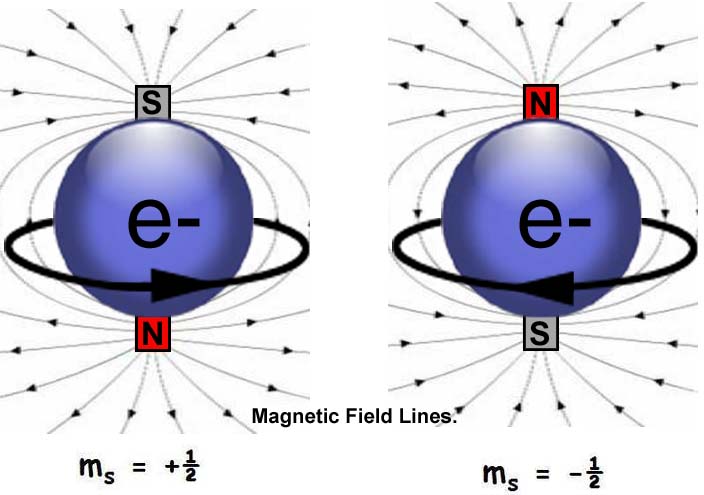
oh and thanks for coming back and looking at it yourself!

in terms of the spin and dividing in half... that's where I go a little off piste, even more, lol and argue that clockwise and anti-clockwise are one and the same thing...
0 -
13 minutes ago, hypervalent_iodine said:2 minutes ago, DrP said:
When numbers get fudged to fit patterns and some kinda cosmic connection is made.... or the numbers don't quite fit so goal posts are moved to make them fit..... usually with some kind of cosmic explanation or conclusion drawn. Or, when a completely innocent series or pattern occurs naturally and just happen to fit a number sequence (why shouldn't it?) and an unnecessary explaination is jumped to... usually involving some cosmic debris - That's numerology in a nutshell - but I'm not an expert.
Maybe you have found some pattern and, as is usually the case in chemistry, there are exceptions to the rule. But it doesn't mean it has a reason... maybe it does has a reason. What do you think is the reason for the run fitting the pattern of prime gaps (saying for arguments sake it did actually fit, ignoring where it doesn't)? (Sorry if you have already said this earlier - I must have missed it or forgot).
To be honest I was going to answer this but the previous:
1. If the diagram is wrong, why bother showing it?
2. What looks right to you?
3. Yes, thank goodness there are / were people out there much smarter than either of us, who knew what they were doing enough to come up with the periodic table!
has put me off...
I would google the no no no no brain and look into concrete thinking version complex thinking... hyper_valent you are a claassic example of 20th Century thinker... lower order thinking using just:
1.Remember
2. Comprehend
3. Apply ...
Unfortunately with the problems facing the 21st century we need more "complex thinkers" who otherwise mankind is finished.
13 minutes ago, hypervalent_iodine said:Quotewhat i will do is go away and find answers to your questions... ;-) because that's what people do that are clever than you Mr Iodine
0 -
Okay cool looks right to me.
Here's a 2D representation of the 3D prime model which is wrong by the way - obviously lol
7 minutes ago, hypervalent_iodine said:Your diagrams are, for lack of a better word, meaningless.
thanks, ;-) feedback is a gift thank god for that periodic table lol and those f orbitals! phew!
-1 -
15 minutes ago, hypervalent_iodine said:8 minutes ago, hypervalent_iodine said:
They aren't fudged. They're placed that way because it's more compact and easier to see. Are you seriously denying the existence of f orbitals?
It is a fudge if you included the 15 elements that are "left out" Hf would sit where Rd is.. and it would be a noble gas lol
f orbitals are just giant s, p and d orbitals anyway
funny how all the probability "shells" are shaped like my diagrams lol those p's look a hell of a lot like what I am showing ;-)
The sequences match, except for that bit in the middle, and please ignore half of the periodic table because those don't match either.
What do you mean the half that barely exists.... and has not been studied in its entirety that half? lol
FYI the half you are suggesting is radioactive: Francium. Firstly, Francium is REALLY rare! It is radioactive and it breaks down into other elements very quickly. It was discovered in France in 1939 by a French physicist called Marguerite Perey. There are thought to be only 30 grams of it on the Earth at one time.
for instance...
8 minutes ago, DrP said:That's Number Wang! Numerology does that a lot.

numerology? lol really...
-1 -
23 minutes ago, hypervalent_iodine said:
It either matches or it doesn't, and in your case, it doesn't. Let's take it further though.
Prime gap sequence:
1, 1, 3, 1, 3, 1, 3, 5, 1, 5, 3, 1, 3, 5, 5, 1, 5, 3, 1, 5
Electronic configuration
1, 1, 3, 1, 3, 1, 5, 3, 1, 5, 3, 1, 7, 5, 3, 1, 7, 5, 3, 1
Again, more problems. 8 of 20 terms don't match. That's almost half. The sequences do not match.
These match (ignore the middle) takes us up to the 57th Element or Ba... row 6! I mean these larger element shells are more fudged even the periodic table fudges at Ba 14 elements aren't included 57 - 71 and 89 - 103
The pattern is a 3D pattern... I'm going to drill into the
There
2 minutes ago, hypervalent_iodine said:Could you possibly respond to the other 95% of my post? The comment about the number 2 was just an aside.
OMG give me a minute! You were wrong about the number 2
Not whoops I'm sorry I made an error no... hurry up answer the more complex bit lol
-1 -
21 minutes ago, hypervalent_iodine said:
Edit: and this is ignoring that you didn't include 2 in your list of primes, despite it being a prime number. As I said, you can't just change the facts the make it fit what you want. If it doesn't fit, it doesn't fit.
Okay this first as it is easier to answer lol... THERE IS NO GAP BETWEEN 1 2 AND 3 THAT IS WHY IT IS NOT INCLUDED
GAP BETWEEN 1 AND 2 = O
GAP BETWEEN 2 AND 3 = 0
GAP BETWEEN 3 AND 5 = 1
HENCE 1,1, ETC
0 -
Yes I know that is the one major problem BUT otherwise, it matches... I did say that from the very first post

it's not my prime gap sequence it is the prime gap sequence lol
2 minutes ago, hypervalent_iodine said:So here's the problem. You have completely ignored the basic tenants of how orbitals are populated to make your pattern fit the order you want it to fit. 4p orbitals do not fill before the 3d orbitals do, so this term should come after rather than before. This makes the sequence 1 1 3 1 3 1 5 3 1..., which doesn't match your prime gap sequence. You can't just change physics to make your pattern work.
On 27/08/2017 at 1:29 PM, danking said:The electron configuration of atoms is a pretty important sequence of numbers:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 (Hydrogen to Palladium) Essentially:
2, 2, 6, 2, 6, 2, 10, 6, 1, 10
If you isolate the up electron spin:
1s1 2s1 2p3 3s1 3p3 4s1 3d5 4p3 5s1 4d5
or:
1 1 3 1 3 1 5 3 1 5 ...
The prime number sequence is an equally important sequence of numbers:
3, 5, 7, 11, 13, 17, 19, 23, 29, 31, 37, 41, 43, 47, 53...
If you isolate the number gaps between the primes i.e. between prime 3 and prime 5 is the number 4 i.e. a gap of 1 number:
Number gaps in the prime sequence:
1 1 3 1 3 1 3 5 1 5
Electrons configuration spin up:
1 1 3 1 3 1 5 3 1 5
There seems to be a correlation between these sequences other than:
1 1 3 1 3 1 3 5 1 5 (Primes Gaps)
1s1 2s1 2p3 3s1 3p3 4s1 3d5 4p3 5s1 4d5 (Electron Configuration from Hydrogen to Cadmium)
Am I missing something here? The prime number sequence and the electron configuration arguably the two most important sequences in the universe and they seem to match?
Is that merely a coincidence or significant?
Thanks for any feedback!
Dan
Here is some of my first post...
I'm not changing physics it is an observation Einstein did mind experiments to come up with relativity - he imagined light lol
I'm showing a DISTINCT pattern - a real PATTERN you can see... with one DIFFERENCE
A CONNECTION between the 2 most important sequences in the universe.
0 -
1 minute ago, DrP said:
Yes it does that - then you delete what you don't want... or you can highlight your quote and then hit the quote button. Both work.
Thanks.. will do...
Just now, hypervalent_iodine said:Okay, lets go through this one thing at a time. The above sequence. Does this show the electronic configuration sequence? Is this the left side of the axis or right?
Okay it obviously gets very small and hard to see but yes...
This is GAPS from - 37 to 37 and this other than the issues raised shows the electron configuration:
5 1 5 3 1 3 1 3 1 1 0 1 1 3 1 3 1 3 5 1 5
These are prime gaps in the sequence
-37 -31 -29 -23 -19 -17 -13 -11 -7 -5 -3 0 3 5 7 11 13 17 19 23 29 31 37
5 1 5 3 1 3 1 3 1 1 0 1 1 3 1 3 1 3 5 1 5
Which takes you from1s2 2s2 2p6 3s2 3p6 4s2 4p6 3d10 5s2 4d100 -
3 minutes ago, hypervalent_iodine said:
PS. It would be useful if you quoted members here using the quote function, rather than copy pasting. At the bottom left of the post you wish to respond to is a Quote button.
I'm trying but on a mac it is VERY buggy and doesn't always work - again this isn't my forum... it copies the entire reply rather than the bits I am trying to answer - maybe 1 question at a time would be helpful
 0
0 -
2 minutes ago, hypervalent_iodine said:
I get that, but you haven't actually addressed my question. If the point is that your prime gaps sequence matches or mirrors your supposed electronic configuration sequence, then please explain where 3, 5, 1, 5, comes from with reference to the latter. I am not interested in the prime gaps component of your post, I am interested in the electronic configuration. Based on how you arrived to your numbers, I do not see how 3, 5, 1, 5, at all fits with the electronic configuration sequence.
I'm trying to answer the questions I can only type so fast and I am having to edit diagrams to repost... I have to also wait 14 seconds lol ( your forum not mine.. )
negative prime numbers are a mirror...
-7 -5 -3 -2 -1 0 1 2 3 5 7
Is that what you meant?
0 -
12 minutes ago, hypervalent_iodine said:
Your pattern doesn't even hold to the d block, so I'm unsure of how it could be consistent through to the f block.
It's not my pattern...
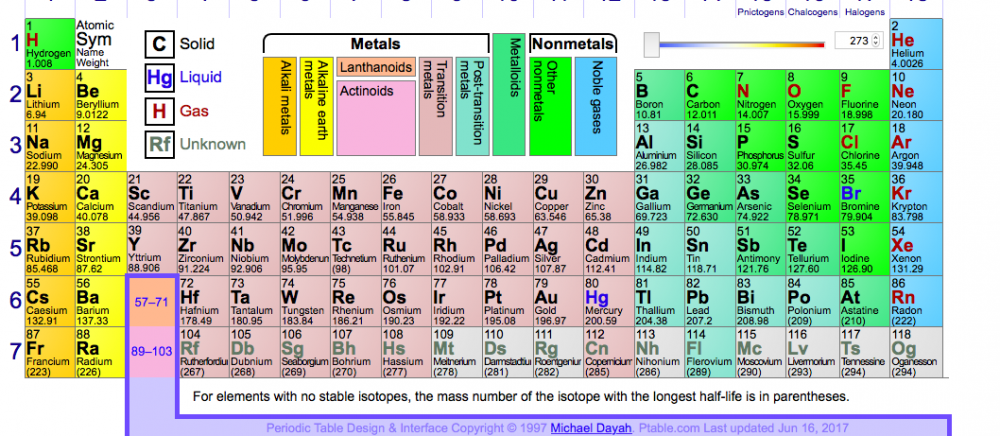
and it does hold and as you stated from f it is pretty much made up anyway! Check out the periodic table and the giant gap from 57 onwards - 14 elements don't fit in...
BUT did anyone shoot Dmitri Mendeleev lol no...
0 -
Apologies re forum this is the first time I have used one... I'm also trying to answer several people at once... and again I'm only posting an observation... it's definitely not made up numbers... just because I am looking at them in a different way doesn't mean it's made up it merely means it's different viewpoint.
Copper doesn't have any 4p electrons, firstly. Even if it did, they absolutely do not get filled up before the 3d's.
Secondly, you claim these two sequences mirror each other, but this only seems to work if you completely make up the last few terms of the electron configuration sequence (which in itself, is made up). Where does the 1, 5 come from? Why is there suddenly another 3 before the second to last 5?
Finally, I fail to see how one made up pattern and another made up pattern that are only partly identical for the first few terms of the sequence has any relevance to, well, anything.
P.S. It's hypervalent_iodine (me) that you replied to, not DrP.
The 1, 3, 5, etc come from GAPS in the prime sequence maths normally deals with DIFFERENCES
gap between 3 and 5 = 1 (i.e. 4)
gap between 5 and 7 = 1 (i.e. 6)
gap between 7 and 11 = 3 (i.e. 8,9,10) etc
hypervalent_iodine
I strongly suggest you check yours first.
You really need to relax... wow.How do you think new discoveries are made? People spot patterns in things other people haven't seen...
0




Primes & Electron Configurations Please HELP! Part II
in Speculations
Posted
Hello,
I posted previously but wasn't brilliantly prepared... I would like to thank those that fed back last time because it has actually really helped which hopefully you can see here - I would really like feedback and if anyone is able to help with the maths that would be amazing and I hope I've addressed the issues raised in the last post. I have created a dropbox folder of higher resolution graphics which I will paste at the end.
It all started when I saw this video: Primes and Twin Primes an awesome journey
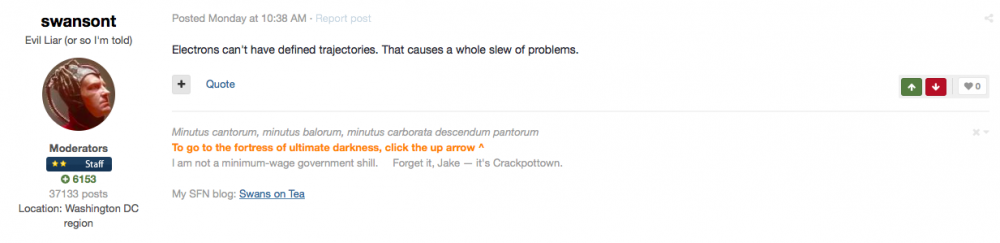
Carlos was trying to find the millionth prime in an effort to win a prize and was using circles to do it… what he didn’t realise was that it was the millionth digit prime number.
He found that if he used circles then he could find primes to the square of the largest circle in the sieve - similar to the Eratosthenes from third century BC.
Here is an image of his work:
You can see that where:
ONLY a circle of diameter 1 & ONE OTHER number crosses the number line that number is a prime so a circle of diameter 3 is a prime…
He found that with a sieve of 142 (largest circle in the sieve is 142) you could “find” primes up to the square of that number 1422 = 20,164
Here’s what that looks like:
Here’s the sieve or the largest circle for just 142….
Here’s what that “data” looks like at larger numbers near the 20,000th
What I saw was that the gaps appeared to correlate with the electron configuration of atoms:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10
Whose electron number sequence pattern is:
2, 2, 6, 2, 6, 2, 10, 6, 1, 10
and for a specific spin (1/2 the sequence above and the relevance I will explore later)
1 1 3 1 3 1 5 3 1 5 ...
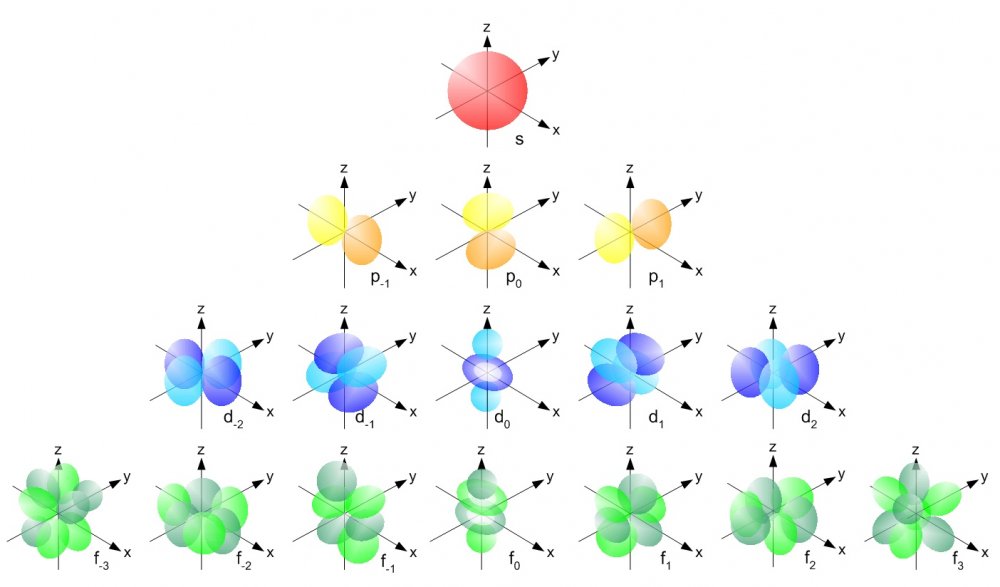
Essentially electrons exist in “shells” called s, p and d etc and each electron is paired (with a few exceptions - cover that later) so electrons have either: UP SPIN ↑ & DOWN SPIN ↓
Based on spin the electrons exist in groups of 1, 3 and 5:
This unusual pattern of 1, 3 and 5 appeared to match the unusual arrangement of electrons for spin up or spin down which is either a coincidence or something more.
If this IS connected I can logically assume that the arrangement would look like this where the spin down is on the left axis and spin up on the right - using Carlos circles for graphical simplicity:
I found that the prime gaps correlated up to a point at number 20 - so the first 40 electrons fit within the prime number sequence - pretty interesting.
But there is a problem with after 20 when the 4p6 and 3d10 are around the wrong way:
Number gaps in the prime sequence:
0 1 1 3 1 3 1 3 5 1 5
Electrons configuration spin up:
1 1 3 1 3 1 5 3 1 5
There seems to be a correlation between these sequences other than:
1 1 3 1 3 1 3 5 1 5 (Primes Gaps)
1s1 2s1 2p3 3s1 3p3 4s1 3d5 4p3 5s1 4d5 (Electron Configuration from Hydrogen to Cadmium)
So does that mean it ends there? no not quite… but we need to better understand how these electrons fill up the shells.
The current rule uses the Aufbau principle / diagram which itself falls down at 23… (https://www.chemedx.org/article/clarifying-electron-configurations)
When the 4s electron moves into the 3d…
and so another rule is used the Rydberg Rule which relies on the spectroscopic data and experimental data:
The Aufbau principle (theoretical model):
4s fills before 3d
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<…..
The Rydberg rule (from experimental/spectroscopic data):
3d can fill before 4s / 4s electron moves to the 3d
1s<<2s<2p<<3s<3p<<3d<4s<4p<<4d<5s<5p<<4f<5d<6d…
Hence at 23 or Cr and Cu:
Cr (4s1 3d5) and Cu (4s1 3d10) where the 3d orbital is filled - or half-filled- prior to the 4s orbital:
At 23 the 4s shell empties into the 3d but why?
Here are all the electron configurations and the red &b blue are where the Aufbau principle falls down:
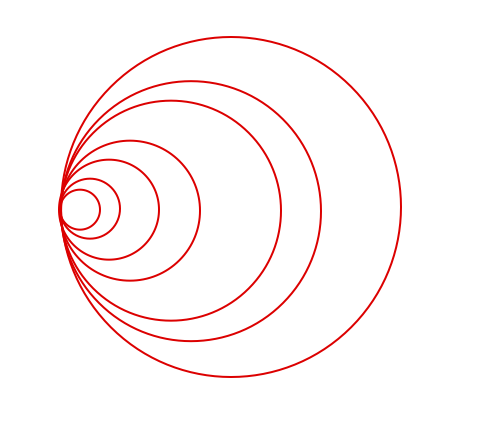
Back to Carlos and the prime gaps - when I looked at his graphics I didn’t see tons of circles but more tons of sine waves…
So I changed his circles into sine waves within a circle or sphere:
Instead of the circle of 2 we now have a wave of wavelength 2 and amplitude 1 (radius of the circle) and a circumference of 3.14
Adding the circles of 2, 3 or 5 we now have a wave of wavelength 2, 3 or 5 and amplitude 1, 1.5, 2.5 (radius of the circles)
And the same for some prime factors turned into waves:
So what happens next?
Well if we use the inverse square law that governs gravity, electrons, light etc as it moves from a source - in this case each time the wave passes the number line is considered a source...
and that wave follows the inverse square law that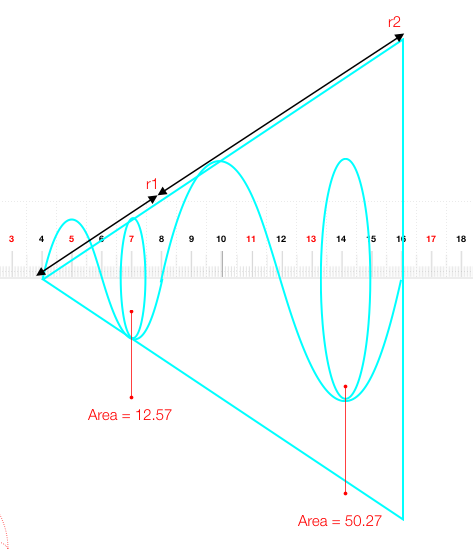
So if 4 is the source then the area of the circle the energy is spread over is 12.57 at r = 1
As the wave at source 4 moves from 8 to point ? the area would need to increase to 50.27 (1/4 reduction in I)
So it would move to 16 and have a radius of 8…
(I've removed the smaller waves that could carry on to keep the diagrams clearer)
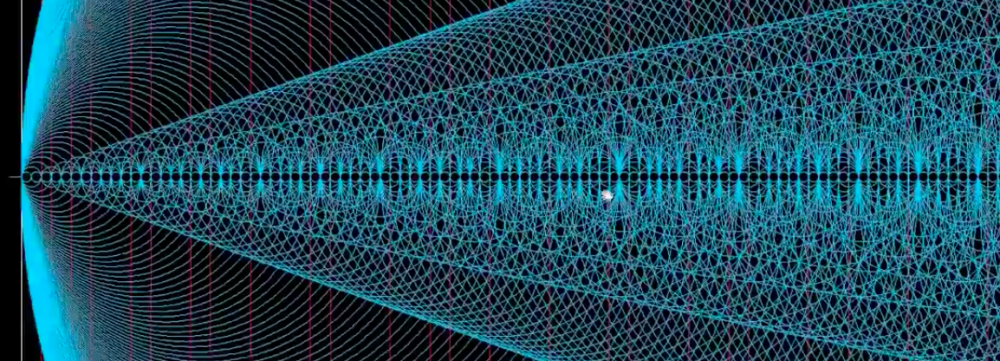
So here are some other prime factor waves growing:
6 (put the electron configuration back in)
8
9
etc
As the source increases in energy the wave increases…
This is all for spin up… what about spin down?
If you take your 4 wave and add in angular momentum and the wave is rotating at the origin:
If you take the wave 4 and rotate it on the origin then at 180 degrees the wave travels back the way it came and moves from 8 to -8 as the circle rotation flips from clockwise to anticlockwise:
So you now have a spin up, spin down for prime factors…
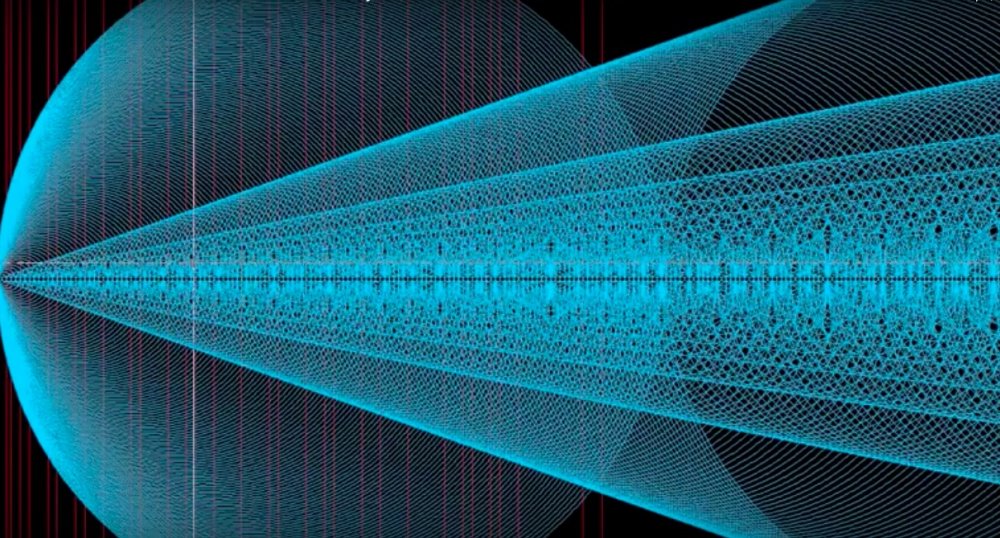
Here’s all the electron waves view:
You can see here that at the 4p and 3d there is “unusual” wave activity and that the 3d could very well fill first before the 4p even though the order is different.
You can also see that a single electro could exist in the 3d shell with a “loop back” at 4s and you can see why perhaps a 4s empties into a 3d.
Not all electron shells are on the same axis:
and if these are “electrons” there are some other elements that are pretty important.
Electrons are in pairs and these pairs are in groups and subgroups which are on a specific axis i.e.
So they don't all weave along the same axis…
BUT the a wave on the x axis would need to create waves that are also on the same axis… so they whole wave needs to be axis specific and these are the different axis.
The s - x axis
The 2p
On the x, y and z axis
The 3d
on the x, y, z and in-between the x, y and z axis
So the wave needs to link the right numbers on the right axis so it needs to be axis specific for the whole wave from source to the largest atom…
If you now add in the axis for each wave i.e. a wave that starts at 6 on one axis would need to cross the number line and be on the correct axis for later electron shells to this formation:
I have not done the f orbitals yet… BUT the 3d have an interesting arrangement with 2 of the 5 coming from previous axis and 3 new axis so you would expect to see 3 new waves at 3d - xy, yz and xz which you do…
What about the actual primes?
So in an electro magnetic filed the magnetic field lines are perpendicular to the electric field:
This is hard to show on Keynote! So JUST the primes 2, 3 and 5
You can almost see the electric waves weave through the prime numbers which are acting as “insulators” like the plastic on a copper wire.
The primes are perpendicular to electric field waves 4 and 6… and the waves “weave” in an out with the primes between 2 field lines.
Apologies for the length... here is the link to the dropbox images: https://www.dropbox.com/sh/ur2da810bv2zjpv/AAAJdhMpa15wIEQnr7s74GuNa?dl=0
Any help with the maths or feedback would be great...
Thanks
Dan King