-
Posts
85 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Everything posted by Mendelejev
-
Well, I've tried it this afternoon with some Bismuth I got from the university labs. I placed it in a flask and heated it quite heavily, but when it was starting to melt, there began to form a sort of crust, gray, not beautiful at all. I stopped heating, and I got some nasty gray powder that don't shine anymore. What did I do wrong ?? Is it the oxidation that was to heavy ?
-
Yes, indeed, the smell was really bad, but the idea of making P from urine and doing an experiment that alchemists did a long time ago, makes it good. But it's not easy to destille urine and you can better do it when your family is on holiday, becouse they don't support the smell
-
Don't forget Sn !
-
Well, I will look for the experiment in my books. Your description is correct but not completeley alchemistic. I have tried it once, but my apparatus exploded, due to over pressure. So I am going to retry it, but more carefully and organised.
-
it's also carcinogenic, so don't ever touch it without gloves or protection. Especially potassium dichromate solutions.
-
LOL !!! Yeah, that would work But, yes indeed, nitric acid can be made by dissolving 2 parts Potassium nitrate (KNO3) in 1 part 98% Sulfuric acid (H2SO4) by volume, and distilling this mixture at 55-60 degrees Celsius until only a white crystalline mass, Potassium sulfate, remains in the reaction vessel. Very pure HNO3 can be made using this method. Or you could make NO2 first. (Nitric acid is made by mixing nitrogen dioxide (NO2), with water. Creating a very pure nitric acid usually involves distillation with sulfuric acid, as nitric acid forms an azeotrope with water with a composition of 68% nitric acid and 32% water.)
-
http://www.epa.gov/ttn/chief/ap42/ch08/final/c08s08.pdf go check this site. But i think it's to industrial. Why do you want to make your nitric acid with ammonia ?? There are several other ways.
-
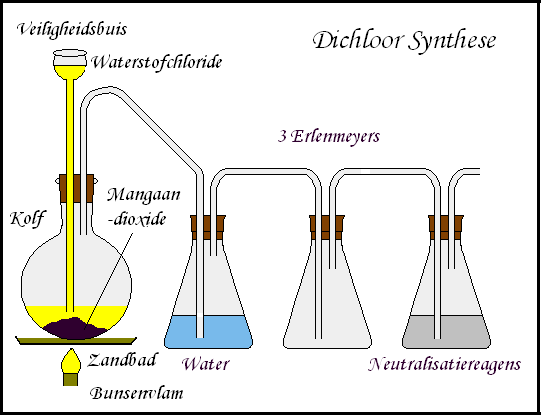
Here you have a picture I made of how I make chlorine gas. Very easy to make. Text is in dutch, but i think it can't be that difficult to translate. When you've made it you should hold a copperwire in the erlenmeyer with chlorine gas. Very nice experiment. When the reaction stops, just place the wire in a bunsen flame a you will have a nice blue color.
-
Read some experiments from alchemists. The isolation of phosphor, for example ! I am going to start the experiment in june. And I am going to do exactly what the alchemists did. Curious if it will work !
-

Who was the greatest scientist or inventor that ever lived ?
Mendelejev replied to vrus's topic in Other Sciences
-
Aren't there some sites with photo's ? Couse it's really difficult to imagine !! And could you also use this method for hydrogen, oxygen, nitrogen, etc. ?
-
I know that terbiumsalts should give a green color and thulliumsalt a blue color. But I don't think it works with the oxides of terbium and thullium. I tried it with my UV-lamp, but it didn't work. Europium(III)oxide (white salt) turned red, but not sooo red. Maybe becouse it's an oxide, and not a complex with big organic ligands that absorb the UV light and give their energy to the europiumcation. I'll try tonight to add a small amount of my europiumoxide into some water or dilute HNO3. I'll tell you my results. Oh would it be possible to change the europiumoxide into a europiumsalt that emits more and brighter red light ? Some kind of simple reaction with the oxide ?
-
I strive for maximum entropy (like nature does). You should see my chamber
-
99,7 % SiO2 Density 2,2 Melting point 2300 °C (Go not above 1300 °C) Same properties as Duran, Pyrex and Simax Duran Pyrec Simax Density 2,23 Melting point 530 °C (Go not above 500 °C) Composition : 81 % Boron oxide, 4 % sodium-potassium, 2 % aluminium oxide SBW Density 2,45 Melting point 555 °C (Go not above 500 °C) Ceran Density 2,57 Melting point 700 °C (Go not above 700 °C) Sodaglass, AR, Soda-potassium Density +/_ 2,5 Melting point 300 °C (Go not above 300 °C) Composition : 74 % silicium, 16 % soda, 9 % calcium carbonate and magnesium
-
So, I put some stars in an erlenmeyer and heat it carefully with a lot of petrol. But what do you mean with 'washes'. Does the zinc sulpide precipitate ?? And what would be better sources ?
-
Hey guys, I am searching how I can make a solution of plastic. I have some plastic stars that glow in the dark, but would like to make a solution of it with the same properties (glowing in the dark). Has someone an idea. I don't even know what kind of plastic it could be. I don't think acids will work. I've tried to melt the plastic. It melts very fast at low temperatures, but loses it's yellowish color and becomes brown, due to combustion i think. And even if I would be able to melt the thing, it would become solid again. And i want a liquid.
-
Lithium cells are very small cells. Perfect for pacemakers ! The cathode is an I2-complex that will reduce to I-. The anode is Li that will be oxidized to Li+. Li+ + e- -> Li E° = - 3,03 V I2 + 2e- -> 2 I- E° = + 0,54 V => 2 Li + I2 -> 2 Li+ + 2 I- The seperation between the anode and the cathode consists of LiI-crystals. Li+ can pass but becouse of the large inner resistance there is no big amount of electricity that can flow threw the cell. For this reason lithium cells will work longer than other cells. Li also has the smallest relative atomic mass of the anode metals, so it can provide the most electrons per mass. This also results in a small weight. But it's quite expensive to produce Li cells out of LiCl-electrolysis. That's why we only use them for some specific applications. (for example pace makers)
-
Each element has a number of isotopes. For example, hydrogen. We know it's hydrogen becouse it consists of 1 proton and 1 electron. Same for iron : we know this is iron and not oxygen becouse it consists of 26 protons and 26 electron (= atomic number). But atoms also have neutrons. The number of neutrons in an atom doesn't have to be the number of protons. For example : hydrogen with 1 proton and 1 electron could also have 1 neutron, but we also find hydrogenatoms with two neutrons (, 1 proton and 1 electron) or hydrogens with 3 neutrons (, 1 proton and 1 electron). Becouse those 3 atoms all have 1 proton, we know they are Hydrogen, but they differ in their number of neutrons. Hydrogen with 1 neutron is called hydrogen. Hydrogen with 2 neutrons is called deuterium. And hydrogen with 3 neutrons is called Tritium. We call hydrogen, deuterium and tritium ISOTOPES of hydrogen. So how do they form ?? Well, just like all the other elements (atoms.). It's not that we can separate the 'normal' atoms from the 'isotopes'. Every atom is in some way an isotope. We just speak of isotopes becouse we find many different atoms for the same element in the periodic table. But they are all formed in the nucleus of stars. First hydrogen and helium will hit each other at very very high speeds and temperatures and form Lithium. This process continues and all elements will be formed. The heavy elements (like Pb for example) can only be formed in super nova's. When those stars explode, the elements formed inside the star are made free, (and can in a second step form our planet earth for example) Of course you have stable and less stable isotopes. That's why we always find a certain percentage of isotopes of an element. Some isotopes are so unstable, they will break down in other isotopes or elements (radioactivity).
-
I just got my UV-torch. It's working quite good. The europium(III)oxide (white powder) turns red. But the color isn't so red. I was thinking, could I use a solvent for my europiumoxide that would cause the liquid to turn red ?? I know europiumoxide isn't soluble in water. But maybe in an organic solvent ? Oh, and you see some purple light when the torch is on. It's quite annoying, becouse you see more purple than red. Could i use something (like a filter) to stop the purple light but not the UV light ???
-
mmm, strange. Couldn't it be an isomerisation reaction ?? That's what I found in my books of biochemistry. But that doesn't explain the fact that it becomes a sirup. The sweetness depends of the sugar you form. So that is possible.
-
It's the World SMALLEST Ultra Violet 12 UV LED Flashlight 1 AA http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=5192580930 But now, you made me curious : why sould i've boughten a 532nm green laser ??
-
Could someone explain me the following : I made a TLC of three amino acids : glycine, leucine and aspartic acid. I used a ethanol-water-ammonia (25%) solution (8 parts ethanol, 1 part water and 1 part ammonia). My calculated Rf values were quite similar with those found on internet : aspartic acid : 0,24 glycine : 0,26 leucine : 0,73 But why does leucine go so high ??? Aspartic acid is the most polar, so i would think it would have the highest Rf value becouse my solution is also polar. Glycine with R = H is less polar, but leucine is quite apolar becouse R = (CH3)2-CH-CH2- ! So it would be logical that leucine would have the smalles Rf-value. Could someone explain me why it's just the opposite of what I've just said ???
-
My biggest mistake in chemistry : I was making hydrogen (2 Al + 6 HCl -> 2 AlCl3 + 3 H2) in a plastic bottle and I had closed it for some stupid reasons. Pressure got to high and when I realised the thing was going to explode, I took it as fast as I could to reopen it, but when I had it in my hands, it was too late. The bottle exploded in my hand. A bottle of 4 mm thick plastic !!! I had to undress becouse the hydrochloric acid was eating my clothes (and me). I couldn't see anything becouse there was so much somke (dark grey smoke). I creeped away, half naked, but got to the door, opened it, and ran away !! This happened on a monday morning, but at 4 o clock I still felt my heart beating like hell
-

Who was the greatest scientist or inventor that ever lived ?
Mendelejev replied to vrus's topic in Other Sciences
What do you think ... DMITRI IVANOVICH MENDELEJEV !!!!!! He will always be my favourite scientist. -
Yes, but ethanol, for example, could create strong intermolecular forces with glycine. even Hydrogen bonds. SO, i still don't understand