

exchemist
-
Posts
3389 -
Joined
-
Last visited
-
Days Won
50
Content Type
Profiles
Forums
Events
Posts posted by exchemist
-
-
2 minutes ago, StringJunky said:
uBlock Origin is available for most platforms. Look in settings as well for more blocks. Ive used it for years.
Thanks. I looked at that but it was not clear to me it can work with Apple Mac OS and Safari.
0 -
20 hours ago, TheVat said:
It's a spectrum. At the high functioning end, one might argue it's just a different cognitive style and I'm open to that. But I worked for a while with people elsewhere on the spectrum, where there were severe cognitive and social disabilities, and for them a cure (or, realistically, any amelioration) would be most welcome.
My son knows several people, formerly at school and now at university, who are on the autistic spectrum and obviously highly capable. The fact - which I had absolutely no idea about - that @Markus Hanke is also on the spectrum (!) is another illustration. I have my suspicions that various eminent figures in history (Newton, perhaps?) may also have been.
People of my son's generation are getting quite comfortable with the idea that autistic people can fit into society perfectly well. But then there are other cases that are clearly very difficult. At the other end of the scale, when my son was small one of his schoolfriends was a little girl who had an autistic brother, who still could not speak at the age of 5. (This girl once told him that when she grew up and married him (!) she wanted 3 children: one boy, one girl and one autistic. )
1 -
7 hours ago, zapatos said:
It seems ads are now showing up in the middle of posts. Ironic that we are not allowed to do so, but SFN does it for us. 😆
I have now experimented with AdBlock Plus, which is free on the App Store, for my iPad. At first it did not do much, but a few days ago I found the app contains an option to permit what it calls non-intrusive ads, which is by default enabled on the grounds that the AdBlock people recognise websites need to make some money. My experience however is that that still permits most of the annoying, moving ads that are so distracting to the reader, e.g. on newspaper websites, to get through. I have now found that by turning that off I can suppress these and my reading experience is now vastly improved. I have the feeling it may also stop the ads coming up in the middle of YouTube videos, though it still allows the ones at the start. I'll give it a few more weeks and if no snags emerge I'll install it on the laptop as well.
1 -
7 hours ago, Externet said:
Hi. Collision avoidance that will not be implemented:
---> https://scrippsnews.com/stories/a-runway-collision-warning-system-for-pilots-stalled-at-the-faa/
This seems written from the point of view of the company (Honeywell), that was and maybe still is trying to promote this apparently expensive and and hard to install system. As I read it, it would only work at 35 airports and would need to be customised for each one. So hardly surprising the authorities did not jump at the chance.
If you read on in the piece, there seem to be less costly alternatives in development.
0 -
1 hour ago, MigL said:
I hate doing laundry, so I take the least amount of time possible, IOW, no pre-soaking; it's a waste of time.
Have three different hampers for colors, whites, and 'it doesn't matter'.
Dump one of them into the washer with appropriate liquid detergent.
Transfer them to the dryer with softener sheet for an appropriate time.
You are done; and if a stain doesn't come out, toss that garment, and learn to be more careful next time.After the washing/drying comes the part I hate even more; folding and putting away.
You've left out the ironing.........
0 -
3 hours ago, Guille Yacante said:
Not anyone can be a scientist. This is only for noble people.
Because in the way of knowledge there are usually lots of times in which one has to recognize he was wrong.
Do we agree?
I'll tell you about my case.
I am 27 years old, I only finished high school, but I have found the cure of autism and a lot of chronic illnesses.
It has always been hidden in plain sight.
Not anyone can be a scientist because intelligence is not innate to everyone.
And now we will wake up to having trusted in white smocks, and in people for their diplomas, for their having been able to repeat well.
And just as intelligence is not innate to everyone so isn't nobility or a good heart.
This way, there are lots of times in which there are perverse people, intelligent by the way, in places of influence.
And these would own big sums of money, would work together, and shape us a system of mental conditioning in which we may think ourselves doing some big job, when it's only part of the biggest lie ever created.
And when any of you be able to realize, which will be your reaction?
Are you going to keep spinning on your hamster wheel? Or help us bring a better world?
Solutions empower the people. Taking away the pacifier may upset some, but when solutions are put at hand, there is not much place for being a pain.
We are learning the lesson worldwide.
Guillermo Yacante Afonso.
Search my name.
Thank you.
I got off my hamster wheel some time ago.
I don't understand the rest of your post at all.
0 -
8 hours ago, swansont said:
As an aside, I have to wonder if the infertility issues getting worse is partly an artifact; infertility clinics cost money, so one wouldn’t expect people to go to a doctor unless they had the means to do something about it, which would increase as household income increased. IOW, we got better at diagnosing the problem, and reduced an economic bias.
It does seem to be a contentious area: https://en.wikipedia.org/wiki/Male_infertility_crisis
Seems doctors don't believe there has been a significant fall in fertility while a number of meta-analysis studies suggest there is at least a fall in sperm count . It may be important that that does not necessarily lead to reduced fertility, provided the count remains above a certain threshold, which apparently it generally does. Curiously, I can find no mention anywhere of antibiotic use having been considered as a possible factor.
0 -
8 hours ago, iNow said:
IF this is true, then two things come to mind:
1) It will dilute the dyes in the fabric and thus cause the colors to fade / become more muddy / less bright and not as saturated and clear.
2) Hydrogen is corrosive (at least it is with metals) and water molecules have two hydrogens so it might weaken and fray the fabric if submerged for extended periods of time.
I defer to our respected chemist members though
As far as I know cellulose, e.g. cotton, is hydrolysed by acid, so by H+ rather than H2O per se.
Wool is keratin, which is a fibrous protein. The amide links in this can also be hydrolysed under acid conditions.
I don't know whether in neutral water either of the these processes occurs at a perceptible rate, but I suppose it is conceivable over very long periods of time. Maybe someone else here knows more about it.
1 -
57 minutes ago, willferral said:
If multiple solvents are mixed together with different evaporation rates will they evaporate together all at the same rate or separately? For example if Butyl Acetate has a evaporation rate of 1. and Acetone has a evaporation rate of 6.1 and ethanol at 1.7 and they are mixed will they evaporate at a uniform rate or will the acetone evaporate first?
If I add a slow drying solvent as a retarder such as Diacetone Alcohol with a .12 evaporation rate will it slow down the evaporation of all the solvents or will they evaporate at the same rate and just whatever amount of Diacetone Alcohol I added will be left behind?
This is assuming all the solvents are miscible and mix together well. Thanks for any help.
If they are miscible, i.e. you don't get a layer of one of them on the surface that prevents the materials beneath evaporating, then to a first approximation they will each evaporate at a rate given by Raoult's Law. This states that the vapour pressure of each component will be proportional to its mole fraction in the mixture. For example if a component comprises 1/3 of the molecules in the mixture, it will contribute a vapour pressure 1/3 that of the pure substance.
There can be deviations from Raoult's Law when the molecules of 2 substances have more affinity for one another than for molecules of their own sort, or conversely when they have stronger affinity for their own sort than for the other.
Here is a link to a fuller explanation: https://en.wikipedia.org/wiki/Raoult's_law. The 2 graphs give you first what happens in an ideal case and then what deviations from Raoult's Law can do.
But in all cases the principle will be that of contributing a vapour pressure (which translates to an evaporation rate) more or less in proportion to how much of the mixture each components represents. N.B. this is on a molar basis (not mass or volume), as it depends of how many of the molecules in the evaporating surface layer are of each substance.
So if you add a miscible slow-evaporating ingredient it will only slow down the evaporation of the other substances according to proportion of them it replaces in the mixture. (The limiting case is adding salt to water. The vapour pressure of salt is negligible. Dissolving salt reduces the vapour pressure of water and thereby elevates the boiling point. But it can only do so to a limited extent because you can't dissolve that much salt in water, so the maximum mole fraction has a limit.)
If however it is a immiscible liquid of lower density, it will float as a layer on the surface and prevent what is beneath from evaporating.
0 -
2 hours ago, Fermer05 said:
Mechanics of comet motion.
Community of Russian scientists. https://vk.com/rosuchA comet is born when a satellite, orbiting a planet during the new moon phase, breaks out of orbit.
Having left the planet's orbit, the satellite moves against the rotation of the Sun, due to which the centrifugal force of the satellite is reduced, and as a result, the satellite rushes towards the Sun.
https://en.m.wikipedia.org/wiki/Comet
If the orbital speed of the satellite is greater than the orbital speed of the planet, then the satellite moves around the Sun in the opposite direction.
https://en.m.wikipedia.org/wiki/Retrograde_and_prograde_motion
In the new moon phase, when the orbital speed of the planet and the satellite are equal, the centrifugal force acting on the satellite from the Sun is zero.
For this reason, the satellites of the planets in the new moon phase approach the Sun.
Perhaps comet Shoemaker-Levy, when rotating around its axis and Jupiter in the new moon phase, approached the Sun, then overturned and scattered into fragments. https://en.m.wikipedia.org/wiki/Centrifugal_force
The eccentricity of a comet's orbit can be expressed using the following formula. E = Vp/Vs.
Jupiter's orbital speed is 12 km/sec.
The orbital speed of Jupiter's satellite Metis is 31 km/sec.
https://www.researchgate.net/figure/Solar-System-Moons-Separations-and-Radii_tbl1_256459609
Metis, synchronously rotating around Jupiter and its own axis at a speed of one revolution per 7 hours, is slowly approaching Jupiter.
And everything that rotates, including satellites, has the properties of a gyroscope, maintaining the vertical position of the axis in space regardless of the rotation of the Earth. https://youtu.be/aj-RClXNloc?si=qOR_20ODcKT-ZmHT
When the axial and orbital speed of the satellite reaches a critical point, the satellite, having the properties of a gyroscope, overturns, due to which the synchronous rotation of the satellite is transformed into asynchronous. https://youtu.be/Lgi9bZ40tHQ?si=yW9VnF3Lj2TqXvAE
During a satellite capsize, a centrifugal force appears, due to which the satellite breaks into fragments, like the Shoemaker-Levy comet.
Next, one part of the satellite fragments leaves orbit and moves around the Sun both clockwise and counterclockwise, another part enters the asteroid belt, and the third crashes into the planet.
https://en.m.wikipedia.org/wiki/Comet_Shoemaker%E2%80%93Levy_9
Perhaps the asteroid belt was formed from the torn apart moons of Jupiter. It is possible that the asteroid belt is located between Jupiter and Saturn.
https://en.m.wikipedia.org/wiki/Asteroid_belt
Asteroids, rotating around their axis and in orbit, periodically collide with meteorites, due to which the asteroid, having the properties of a gyroscope, first sways due to a violation of the center of mass, and then overturns, leaves orbit and moves towards the Sun. https://youtu.be/1n-HMSCDYtM?si=CWN0ToKqhw8eOFB3
The claim that tidal forces tear apart comets is questionable. Because the tidal force is too weak and depends more on the diameter of the comet than on the distance from the Sun to the comet. https://en.m.wikipedia.org/wiki/Tidal_force
The stability of the orbits of planetary satellites is also reduced by unstable orbital resonance and stable orbital resonance between the Sun and the satellite.
Perhaps the supermoon is the result of a stable orbital resonance.
The supermoon also depends on the orbital speed of the Earth and the Moon and the shape of their orbits.
Perhaps the gyroscope has other unstudied properties, one of them is the Dzhanibekov effect.
The above can be easily verified by experiment. https://en.m.wikipedia.org/wiki/Orbital_resonance
Anticyclones also have gyroscope properties, due to which anticyclones are blocked.
http://meteoweb.ru/2018/phen20180730.phpContinuation: Forum of Akademgorodok Novosibirsk. The science. https://forum.academ.club/index.php?showtopic=1235578
Astronomical forum "AstroTalk". https://astrotalk.ru/viewtopic.php?f=5&t=10510Identical rubbish previously posted elsewhere and shut down, so now it pops up here.
0 -
14 minutes ago, swansont said:
If it were permanent we’d probably notice the correlation.
There have, I believe, been reports of reduced sperm count in industrialised societies and some discussion about what might be responsible. Things like endocrine disruptors get mentioned but so far as I know nobody has fingered the widespread prescribing of antibiotics.
0 -
33 minutes ago, Carrock said:
For something like the Voyager grand tour, less than one a century...
For a slow journey, all you need is the delta v to get to the first flyby and for course corrections; any asteroid chosen with this in mind would have frequent low delta v options, I'd guess one every year or two. Planetary alignments, possible journeys and required delta v would likely be all worked out long before any mining.
There would be a balance between the cost of rocket fuel etc to minimise journey time and the cost of mined resources unavailable during the journey.
NASA manages these flybys regularly and, it seems, generally chooses to keep time to ultimate target at less than ten years.
I had an idea it might be something like that. So then the issue would be whether a commercial scale mineral transport operation could function with such long intervals.
0 -
2 hours ago, Carrock said:
and similar comments...
There are a lot of issues with mining asteroids but I don't think this one is significant.
All the delta v you need is sufficient to arrange a first planetary flyby, with slowing and deflection towards another flyby planet, basically the reverse of the many flybys used to get spacecraft from earth to e.g. Jupiter. The minerals would also need entry protection to survive entry at somewhat more than earth's escape velocity.
e.g. a package which would miss a planetary flyby by ten million miles in 10 years' time would only need a delta v around 100mph.
OK, Interesting. Roughly how many of the requisite planetary alignments would there be per year, or per decade?
0 -
7 minutes ago, joigus said:
Schizotipical behaviour is not to do with mental stability. It's to do with delusional perception of experience (sensory or otherwise). Have you skimmed through the wikipedia article or references thereby? My emphasis in boldface in a sample from mentioned article:
Etc.
But you still stigmatise them as ill. So ill but stable?
0 -
1 hour ago, TheVat said:
I've heard history buffs say that Luther and the Reformation wouldn't have happened without the invention of the printing press. (which wasn't solely about the written word - Luther also used woodcuts, to present simple stories to those less literate) I really can't think of anything that wasn't advanced by the press, given its role in dissemination of information and promoting knowledge. It eventually shifted literacy from a tiny elite to a majority of the population. Sure, it was double-edged - easier to spread propaganda and libel, too - but what technologies haven't had a double edge at some point? Societies that do well have information gatekeepers who filter out the lies, nonsense, sophistry, etc. The US Supreme Court just heard oral arguments yesterday on litigation over what such gatekeepers should do in social media companies.
Yes the printing of the bible in the vernacular was a significant milestone, democratising the process of reading and interpreting the bible - and thereby to some degree disempowering the clergy. The printing press democratised knowledge of all kinds, a process the internet is taking further today - with even fewer controls on quality.
1 -
1 hour ago, user101 said:
The actual example was in Hebrew.
But the above example is in English ( just an example showing how the numbers and letters are assigned).
Ah Hebrew.
Why don't you put in "ballocks" and see what matches you get? That's the sort of thing I would do to demonstrate what rubbish it all is. Have you tried something like that?
0 -
1 hour ago, user101 said:
I'm currently doing a little research on Gematria. Would like to hear some opinions from the scientific community.
Gematria Brief Intro:
In its simplest form, gematria involves assigning a numerical value to each English letter. The numerical values are assigned based on the order of the letters in the English alphabet. For example, the letter A has a value of 1, the letter B has a value of 2, and so on.
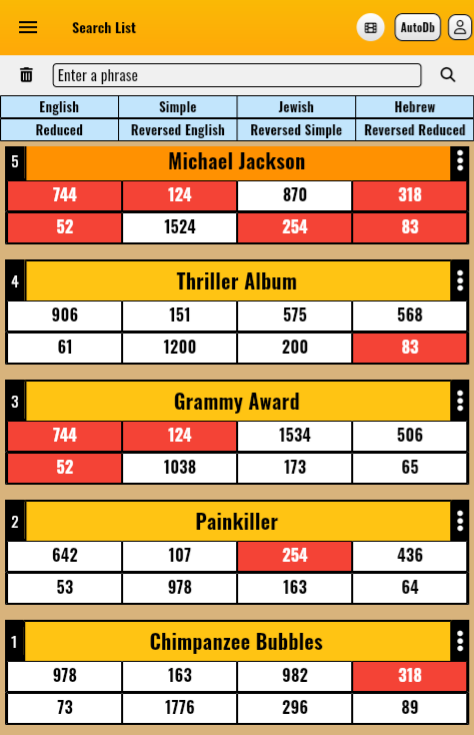
Gematria can be used in a variety of ways. It can be used to find hidden meanings in the Bible, For example, the name 'David' has a numerical value of 14, which is the same numerical value of the word 'king.' This suggests that David was destined to be king, In general, any words or phrases sharing the same numerical value are considered potentially connected and containing hidden meaning.
You can read more about it here : Gematria Wikipedia | Gematria History And Its Present Usage
Modern English Gematria Usage:
Example 1: Entering the phrases "Thriller Album," "Grammy Award," "Chimpanzee Bubbles," and "Painkiller" into a Gematria Decoder reveals that they are all connected with "Michael Jackson."image showing "thriller album", "grammy award" and more has matching values (in red color) with "Micheal Jackson"
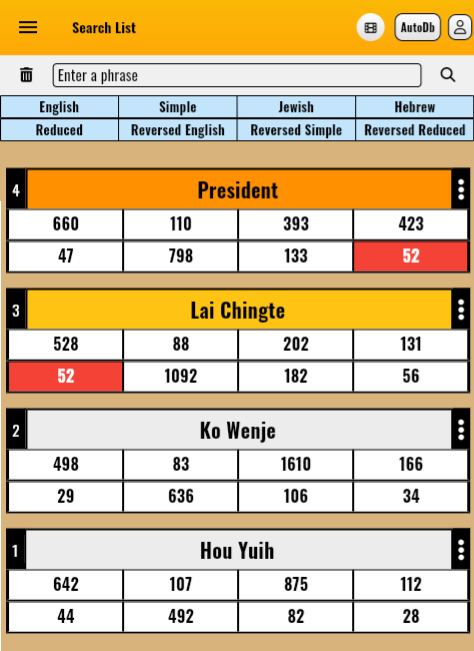
Example 2: In the 2024 Taiwan presidential election, there were three candidates: Lai Ching-te, Hou Yu-ih, and Ko Wen-je. Entering their names into the Gematria Decoder revealed that only Lai Ching-te has a connection with the word "President," and indeed, Lai Ching-te emerged as the winner and became the President of Taiwan.
If these results are pure coincidental, why are there so many relevant matches?
Here are the questions:
1. How do you explain this phenomenon from the perspective of probability and coincidence?
From the perspective of true believers, a single gematria value can be associated with many words and phrases. Making sense of them all involves connecting the relevant words and filtering out those that aren't relevant. This process requires strong intuition, knowledge of the subject of interest and unconventional thinking to establish connections and unveil hidden meanings.
2. How do you explain to true believers that their train of thought on gematria is seriously flawed?
I’m confused by this. How do you get 14 out of the letters in David? Isn’t v alone 22?
0 -
40 minutes ago, Airbrush said:
Very interesting. I like your explanation. Then you said that "something" maybe some kind of sub-nuclear particles possessed the energy. Let's work backwards. Now we have matter. Before that, matter was in the form of energy, sub-nuclear particles possessed energy, and we don't know what was between that and the start of the big bang. Right?
No matter was not "in the form of energy". That is the same confusion as before. There would have been radiation and fields (radiation is a form of oscillating field) that possessed energy. The entity is the radiation, or the field. Energy is one of its properties, along with other properties like direction, phase, frequency, amplitude and so forth.
But my very limited understanding of this (I'm not even a physicist) is that when you try to extrapolate back you reach a limit at which our current theories of matter, radiation and fields etc break down. So we can't "see" any further back, even theoretically. Strictly, the big bang theory starts from the limit of credible extrapolation. All the stuff about singularities etc only has the status of conjecture, so far as I know.
2 -
23 minutes ago, Airbrush said:
Thanks for your explanation. But what if the "surface" or skin of the balloon, had a thickness of over 100 billion light years? Then the expansion would resemble what we can see, no voids would be seen, and we would not know the direction of the expansion.
Are you suggesting that the big bang was not an expansion of energy? First there is energy. Much later, after it cools down, a huge amount of energy congeals into a small amount of matter, E = mc2, so E/c2 = m.
Yup, that’s a (quite common) misconception. We can only speculate about what might have been first, but what’s for sure it wasn’t just “energy” somehow existing on its own. That would be as silly as claiming that what came first was “momentum”, without saying the momentum of what. You can’t have a jug of energy any more than you can a jug of momentum, or velocity. All these are properties, not entities.
Incidentally, “m” in Einstein’s equation does not stand for matter, it stands for mass, which, like energy, is a property of matter, not a free-standing entity. Another misconception is that the equation predicts “conversion” between energy and mass. What it actually says is that rest mass has energy. It’s not one or the other but both at once. The entities involved are radiation and matter. Energy and mass are properties.
In the early stages of the big bang model, there is thought to have been radiation, and sub-nuclear particles, I think. It will have been these entities that possessed the energy.
1 -
48 minutes ago, GeeKay said:
I read somewhere that a single 30m wide near-Earth asteroid (2012-DA14) may be worth some $20 trillion dollars in terms of its rare metals content. Meanwhile, the global precious metals market in 2022 was worth $290 billion dollars. That's approximately one hundredth of what DA14 could offer the world. Does this mean then that the mining of one small run-of-the-mill asteroid every few years would be plenty enough to satisfy Earth's needs for the foreseeable future? In other words having an entire offworld mining industry with fleets of asteroid mining outfits plundering the Main belt for its platinum-group metals etc, etc, would be superfluous. . . strictly for the birds, in fact?
Of course, this doesn't factor in surveying costs, those required by heavy plant infrastructure, transportation costs, risk factors and all the other negatives that must otherwise weigh down such an enterprise. Even so, just for the sake of argument - assuming, for instance, fusion-powered spacecraft are running the show by now instead of chemical rockets - would the above thought experiment still hold some water here?
PS. I've posted this question here in the Lounge, given that its topic addresses mineralogy as well as astronomy. Couldn't think where else to put it.
Correction: the ballpark figure quoted above should be one 69th rather than a 100th. My apologies.
My understanding of this subject is that the killer, economically, is the huge cost of the change of momentum required to bring extracted minerals back to Earth. These asteroids are on a very different orbit from that of the Earth and momentum change (rocket power) is very expensive, per kilo of payload.
By introducing fusion as a technical mcguffin to overcome that obstacle, it seems to me one is already making the exercise so far from practical reality as to have little meaning. It then risks turning into one of those "What if the sky were made of concrete?" questions.
0 -
18 hours ago, KJW said:
If pressure is independent of non-translational motion, and temperature is directly proportional to pressure, then temperature is independent of non-translational motion.
One thing that was never made clear throughout this discussion is what temperature is. I felt that temperature was being conflated with energy. But I will agree that my view was somewhat perverse, and I'm not even sure how it arose. It is correct for gases, but questionable for solids and perhaps liquids as well. Each degree of freedom has an energy of ½kT. Therefore, temperature can be regarded in terms of the energy of a single degree of freedom. And the equipartition theorem ensures that the single degree of freedom can be any of the degrees of freedom, not just translation. This seems to be the argument against me. My argument was basically that it isn't all the degrees of freedom that contribute to temperature because it is only one degree of freedom whose energy specifies the temperature. But translations are quite ubiquitous, and the three translation modes always occur together. So instead of temperature being specified in terms of one degree of freedom with energy ½kT, temperature could be specified in terms of the three translational degrees of freedom with energy 3/2kT. And given that only translations contribute to pressure, it would seem that translations are quite special compared to the other degrees of freedom.
However, I am also aware that temperature emerges as a Lagrange multiplier in statistical thermodynamics, so I'm not entirely wedded to the ideas I expressed in this thread.
Well temperature is proportional to energy so in a way it is just a matter of choice of units whether one talks about temperature or energy in this context. To my way of thinking the distinction between the role of modes is not real, since all degrees of freedom that are excited (at NTP in gases vibrational modes generally aren't) contribute 1/2kT each to the overall energy - which means temperature, in effect. Yes, pressure is proportional to the temperature (or energy) in the translational modes, but it is also proportional to that in the non-translational modes too, as they are all equal.
One test of the idea that the translational modes are special might be if one could make a case that the flow of heat is transmitted only through translational motion. I am sceptical, since the modes all exchange energy.
1 -
1 minute ago, cryptocracy said:
25 to 45 age group. I will research your suggestions and edit the video.
That reply makes no sense.
0 -
1 hour ago, cryptocracy said:
Hello friends, I have a small YouTube channel. I share videos about science. Can you please suggest me a video topic?
What would be the level of knowledge of your intended audience?
0 -
The Shadow of the Wind, Carlos Luis Ruiz Zafon, in English translation.
0

Are the negative effects of antibiotics on male fertility permanent?
in Medical Science
Posted · Edited by exchemist
Yet clearly it has little long term effect in practice, since amoxicillin is widely prescribed (in the US, as much as 24% of all antibiotic prescriptions:https://www.definitivehc.com/resources/healthcare-insights/most-prescribed-antibiotics) and we do not see any mass outbreak of sterility in the population.