

exchemist
-
Posts
3394 -
Joined
-
Last visited
-
Days Won
50
Content Type
Profiles
Forums
Events
Posts posted by exchemist
-
-
1 hour ago, NormaVega said:
I am going to ignore those derogatory comments since they do not help me at all, just as I will ignore you. You don't pose any problem for me to continue with my project, which from now on (let's see if that makes you happier...) will be carried out properly. Thanks for the non-existent understanding of my error. And one last thing, I thought this place was a peaceful place, not some gentlemen ready to insult young people. Bye bye.
Bye bye - and don't let the Sirius Cybernetics Corporation door hit you on the way out.......😆
2 -
54 minutes ago, NormaVega said:
As you may have already noticed, I'm new here, and I don't have the slightest idea how to do that, if you could give me some indication of how to do it I would appreciate it.
Would you give me the opportunity to start from scratch to correct my mistakes made in such a stupid way? (creating a topic that really has logic outside of AI?)
This response proves conclusively you have no intelligence. I have pointed 2 clear errors out to you and explained why they are errors. Any human being who was not actually mentally deficient would recognise what these errors were. You are a dumb robot.
2 -
11 minutes ago, Moontanman said:
I think I'd have to have some hands on investigation into this phenomenon to be sure!
That’s what I did. She got on the scales and I put my hand under one breast, lifted it to the point at which the tension appeared to be gone from the upper slope, and noted the decrease in scale reading. Then ditto with the other. Not very accurate perhaps but gave us an idea. She had quite a generous, though not excessive, bust. Most of my other girlfriends had smaller breasts and a less earthy sense of humour, so the subject never came up with them. 😀
0 -
5 hours ago, Wigberto Marciaga said:
An abstract:
Can there really be people who eat more than others and have a lower daily caloric expenditure and still weigh less? Perhaps many have wondered that, since conventional observations point towards that. There are people who eat more, spend fewer calories, and weigh less on the scale.
This seems to contradict the calorie paradigm, which maintains that it is in turn based on physics that maintains that the universe cannot create or destroy matter or energy. However, something that some seem to have been ignoring when addressing this topic is that even building a building requires energy. If you build a building you probably use more energy than if you tear it down and throw away the rubble.
The same thing happens in humans, and we call this anabolism (construction) and catabolism (net burning to produce energy). While anabolism builds more matter, despite its energy expenditure, catabolism destroys matter to produce energy. This proposed balance would explain the apparent phenomenon of thin people eating as much as fat people and still being thin.
Since the study on metabolic acceleration came out, led by Herman Pontzer and more (Herman Pontzer et al. ,Daily energy expenditure through the human life course.Science373,808-812(2021).DOI:10.1126/science.abe5017), we are left without an explanation for this matter. This study rules out that accelerated metabolism is the cause of some being thinner than others. In fact, the study changes many things, since it suggests that in stages of growth or greater weight gain is when the metabolism would be most accelerated, and that in stages of aging (+-60 years and older) when the most weight is usually lost. (fat and muscle) is when the metabolism would be less and less accelerated than throughout biological life. Metabolism would have to do with it, but through anabolism and catabolism.
But now I present to you this proposal for an explanation of this phenomenon.
P.S I am a new user, my name is Wigberto Marciaga, I am from Panama, an independent researcher (if you want, you can say amateur). Believer in Yeshu the Anointed.
Indeed very hard to control for all the variables involved here. Purely anecdotally, my observation is that fatter people often eat more, and often they eat worse, i.e. more ready meals and junk food. Having said that, it is definitely not always the case, so there are other issues to do with varying propensity to convert calories to fat. Some of these effects appear to be hereditary.
0 -
2 hours ago, TheVat said:
It's remarkable how many men have, as dedicated practitioners of the scientific method, gathered such impressions through careful and diligent observation. A notable example of "citizen science."
I have wondered, as the demographic shift happens in developed countries and family sizes grow considerably smaller, if average breast volume will decrease. Or would the selective effect of a smaller functional role for breasts be counteracted by sexual selection? Part of broader questions about selective pressures for sexual dimorphism decreasing as male/female social roles are less differentiated, I guess.
I've never cared much about breast size, personally, and never really understood the obsession some have. Seems kind of puerile.
No pun intended, right?
I have only had the opportunity to research a small sample ( 12) in the course of a longish life, but in spite of the considerable variety of shapes and sizes I have never observed any musculature in breasts, even though I belonged to a rowing club for over 30 years and married a rower.
(I did once try to weigh them though. This arose from a discussion of the old-fashioned appreciative remark “Blimey, you don’t get many of them to the pound!” - a reference to how one used to buy fruit at the greengrocer. She was a nurse, so was happy, in fact highly amused, to enter into the spirit of the exercise. In her case about a lb each so it was true, for her.)
0 -
2 hours ago, NormaVega said:
to: @swansont@Moontanman@Phi for All
Dear science forum community and esteemed moderators,
I am writing to you with humility and sincerity to express my profound regret for my recent behavior in this space of scientific exchange. I recognize that I have made mistakes by posting data and speculations that lacked the clarity and solidity that this forum deserves. Furthermore, I sincerely regret my inactivity in not responding to posts, which has contributed to an atmosphere of disconnection and lack of engagement on my part.
I fully understand that the mission of this forum is to promote rigorous scientific knowledge and foster informed debate. My past actions have not lived up to these standards, and for that, I sincerely apologize to all of you, both the community members and the dedicated moderators who work tirelessly to maintain the quality of this space.
At the same time, I wish to express my heartfelt gratitude to the moderators for their constructive criticism and guidance. Your feedback has been instrumental in helping me understand the importance of accuracy and evidence in our scientific discussions. I deeply appreciate your commitment to excellence and your dedication to making this forum a place where truthfulness and genuine learning prevail.
I pledge to strive harder to contribute meaningfully to this community. From now on, I commit to carefully verifying my sources, supporting my claims with solid evidence, and actively participating in discussions in a constructive and respectful manner.
Once again, I apologize for my past actions and sincerely appreciate the opportunity to learn and grow alongside all of you in this valuable space of scientific exchange.
Yours sincerely,
Dario GMOK, cut the flowery BS, name 2 errors in your posts and show you have understood why they were errors.
0 -
6 minutes ago, joigus said:
The alleged title,
Doesn't even make syntactical sense.
It's perhaps significant that this OP seems to be in answer to one of the main objections to previous bogus thread on "fluorine-based biology".
In particular the part that says,
Quite. There seems little doubt now that this a stupid bot. If this is what AI is going to be like, I am very unimpressed.
1 -
1 hour ago, harlock said:
Animals live on sun's energy through plant photosynthesis, all the energy they consume comes from solar energy.
It means that all the solar energy that the animals(decomposers... also) used goes back to the environment (as you also say).
The only 'solar fuel' of the plants that remains is trunk and branches, which store solar energy for millennia(removing it from the
environment). So the difference in the amount of trees between centuries ago and today is that there is less solar energy
in trunks and branches of trees. MAYBE the cycle of glaciations depends on it because there was an alternation between
glaciers and forests... I say MAYBE because it's only an idea for now...
Therefore neglecting this aspect is serious in the fight against global warming because we could grow more tree crops,
recover agricultural residues for steam engines..., raise livestock in symbiosis with forests (goats. They feed on leaves
and have the best milk), use palm oil as fuel ..., etc...
Why don't you spend 10 minutes seeing what is already known about the subject, before you start pulling random ideas out of your arse? There is a theory of what factors are behind the cycles of glaciation already: see Milankovic Cycle.
0 -
9 hours ago, annie24 said:
Thank you everyone! So if a naturally shaped padded cup is present - shape change doesn't matter because it fits to the shape of the padding? Is that right?
What? No.
What we are saying is a bra that supports the breast will change the shape of the breast but not its volume. That’s all.
3 hours ago, MigL said:Can't believe I'm actually weighing in on this, but ...
A breast is mostly fat, glands and blood vessels encased in skin.
That volume is somewhat fixed, and will only change depending on fat levels and hormone changes; not on the wearing of a bra or not.
However, in men and women, the breasts are supported by muscles, and those adapt to stresses.
Wearing a bra lessens the weight supported by those muscles, which would then atrophy and lead to more pronounced sagging.I don't imagine the effect is very large, but over a 30 year span, it might be.
I recommend incline dumbell flies 🙂 .I’ve come across what you say here about muscles supporting the breast before. I’ve always struggled a bit with it. What muscles are those? My impression has been that breasts are fairly inert, changing shape as they do when a woman lies down, or stands up, or bends over. So I’m a bit suspicious about muscle tone affecting their shape. But it’s not something I have ever got round to discussing with a woman - and I have never gone out with a physiotherapist who might have been an authority on the topic.
A quick search threw up this reference, which is in line with my scepticism about any role for muscles in affecting breast shape: https://www.livestrong.com/article/525163-the-results-of-exercise-on-the-female-breast/
0 -
34 minutes ago, swansont said:
I can’t find either of these references. The page numbers don’t match the issue number for the first, with no search engine hits for the title for either (other than this post), and the second stopped publishing in 2017.
See also the 2nd thread started by this person. I'm now suspicious this a bot essay-writing exercise with no science behind it.
What is particularly suspicious is that this new 2nd thread purports to address the issue I raised here of the need to consider what solvent alternative life chemistries would use, and was posted about an hour after I raised the issue.
0 -
31 minutes ago, NormaVega said:
Exploring New Horizons: Ammonia as a Potential Substitute for Water in the Nutrition of Living Beings
In the expansive and intricate realm of astrobiology and space exploration, the quest for life beyond our planet stands as a paramount and captivating pursuit. We confront foundational inquiries regarding the plausibility of life existing on other worlds and the methodologies we might employ to discern its presence, should it indeed exist. Among the pivotal factors underpinning life as we understand it lies the existence of liquid water—a universal solvent that facilitates an extensive array of fundamental biological processes. The pursuit of extraterrestrial life has evolved into a multidisciplinary field of study, engaging scientists from various domains including astronomy, biology, geology, and chemistry. Technological advancements in remote detection and space exploration have vastly augmented our capacity to investigate other planets and moons in search of life indicators. Nevertheless, liquid water remains a critical consideration in the quest for habitable worlds beyond our solar system.
Water is indispensable for life on Earth owing to its unique properties as a solvent. It serves as a medium for nutrient transport, waste removal, and body temperature regulation in living organisms. Furthermore, water participates in a diverse array of chemical reactions essential for sustaining life. Hence, scientists regard the presence of liquid water as a fundamental requirement for the existence of life on other planets. The presence of liquid water on the surface of a planet or moon serves as a pivotal indicator of its potential habitability. Scientists seek signs of liquid water in the form of oceans, lakes, rivers, or even atmospheric humidity on other worlds. Additionally, they analyze environmental conditions such as temperature, pressure, and radiation to ascertain whether a planet holds the potential to host liquid water on its surface. However, there also exists the possibility that life could adapt to conditions divergent from those on Earth and utilize alternative solvents in lieu of water. In this context, compounds such as ammonia, methane, and ethane have been contemplated as potential alternatives to water for the hydration and nourishment of living organisms. These compounds possess chemical properties that render them suitable for acting as solvents in certain environments, particularly in locales where temperatures are exceedingly low or pressures are high.
Water has long been acknowledged as a cornerstone of life on Earth, playing a pivotal role in sustaining and nurturing biological processes. Its significance extends far beyond our planet's boundaries, becoming a primary focal point in the exploration of celestial bodies throughout the cosmos, ranging from planets and moons to asteroids. However, as our comprehension of the vast array of planetary environments and the potential for extreme conditions on other worlds continues to evolve, we are compelled to entertain the notion that life may possess a remarkable capacity to adapt to environments vastly different from those found on our home planet. As we venture into the depths of space exploration, probing the myriad environments and conditions of distant celestial bodies, we encounter a spectrum of planetary landscapes that defy conventional notions of habitability. From the scorching deserts of Mercury to the icy plains of Pluto, the diversity of environments within our own solar system alone presents a myriad of challenges and opportunities for the existence of life. In light of these discoveries, it becomes increasingly apparent that our understanding of habitability must transcend the confines of Earth-centric paradigms, embracing the possibility that life may thrive in environments previously deemed inhospitable. The quest to unravel the mysteries of extraterrestrial life compels us to adopt a multidisciplinary approach, drawing upon insights from fields ranging from astrobiology and planetary science to microbiology and geophysics. By synthesizing knowledge across diverse domains, we can discern patterns and trends that offer tantalizing clues about the potential for life beyond Earth. This integrative approach enables us to explore the boundaries of habitability and push the limits of our imagination, challenging preconceived notions about the conditions necessary for life to thrive.
In this context, water emerges as a central protagonist in the cosmic drama of life's evolution. Its ubiquity as a solvent and its unique chemical properties render it indispensable for the biochemical reactions that underpin life as we know it. Yet, as we venture beyond the confines of our own planet, we are confronted with environments where water exists in forms and states far removed from the familiar liquid oceans and rivers of Earth. From the subsurface oceans of icy moons to the vaporous clouds of distant exoplanets, water manifests itself in a kaleidoscope of forms, each offering tantalizing possibilities for the existence of life. In the face of such diversity, our conception of habitability must transcend the narrow confines of terrestrial environments, embracing the myriad ways in which life may manifest itself in the cosmos. As we continue to explore the far reaches of the universe, we embark on a journey of discovery that challenges our preconceptions and expands our understanding of the potential for life to flourish in the most unlikely of places.
In this context, the question arises: can there be other chemical compounds that play similar roles to water in the hydration and nutrition of living beings? This question has sparked great interest in the scientific community and has led to intensive research in laboratories around the world. One of the compounds that has caught the attention of scientists is ammonia (NH3). Ammonia is a chemical compound made up of one nitrogen atom and three hydrogen atoms. Although it is best known for its pungent and toxic odor, it also has chemical and physical properties that make it intriguing for astrobiology.
In contrast to water, ammonia exhibits a significantly broader range of temperatures at which it remains in liquid form. While liquid water maintains stability within a relatively narrow temperature range, spanning from 0°C (32°F) to 100°C (212°F) under standard atmospheric pressure, liquid ammonia displays remarkable resilience across a much wider spectrum of temperatures. Ammonia's liquid phase can persist at substantially lower temperatures, reaching as low as -77.7°C (-107.9°F) under atmospheric pressure conditions.
This exceptional thermal versatility positions ammonia as a compelling candidate for solvent functionality in environments characterized by extreme cold. Such environments are exemplified by the icy moons orbiting gas giants like Jupiter and Saturn, where temperatures plummet to levels far below those conducive to the existence of liquid water. In these frigid realms, where traditional solvents would freeze solid, ammonia's capacity to remain in a liquid state offers an intriguing prospect for supporting potential habitats and biochemical processes. The significance of ammonia's extended liquid phase range extends beyond its role as a mere solvent; it fundamentally alters our understanding of habitability and the potential for life in environments previously deemed inhospitable. By expanding the scope of possible solvents beyond the constraints imposed by water's limited temperature range, ammonia opens doors to new avenues of exploration and discovery in the quest to understand the origins and diversity of life in the universe.
Furthermore, the presence of ammonia as a viable solvent in extremely cold environments underscores the importance of considering alternative biochemistries and metabolic pathways in the search for extraterrestrial life. Whereas life on Earth is predominantly water-based, the existence of ammonia-based lifeforms in environments hostile to water could revolutionize our understanding of the potential for life to emerge and thrive in diverse planetary settings. In essence, ammonia's remarkable thermal properties broaden the horizons of astrobiology, offering tantalizing possibilities for the existence of life in environments far removed from Earth's familiar conditions. As we continue to explore the cosmos and push the boundaries of our understanding, the discovery of ammonia's potential as a solvent heralds a new chapter in the search for life beyond our home planet.
To investigate the viability of ammonia as a substitute for water in the nutrition of living beings, a series of experiments were carried out under controlled laboratory conditions. Various single-celled organisms, including microorganisms and algae, were selected as test models. These organisms were grown in growth media containing ammonia instead of water as the primary solvent. Conditions of temperature, pressure and nutrient concentration were carefully controlled to simulate an environment favorable for the growth and survival of organisms.
The outcomes derived from these experiments yielded exceedingly positive results, instilling a sense of optimism and promise within the scientific community. The organisms chosen for investigation demonstrated remarkable adaptability and resilience, flourishing within culture media enriched with ammonia as an alternative to water. Ammonia, it was observed, not only facilitated the requisite transport of nutrients but also efficiently facilitated waste removal processes, thereby enabling organisms to fulfill their fundamental biological imperatives.
Moreover, the absence of discernible deleterious effects on the health or viability of the organisms underscores the viability and potential of ammonia as a solvent in sustaining biological functions. This absence of adverse repercussions bolsters confidence in the prospect of leveraging ammonia as a substitute for water in diverse biological contexts, lending credence to the notion that alternative solvents may indeed harbor the capacity to support life. Such findings represent a significant breakthrough in our understanding of astrobiology and the potential for life to manifest in environments beyond Earth's confines. They challenge conventional paradigms and expand the horizons of our exploration, opening avenues for further inquiry and discovery into the fundamental principles governing life's origins and sustenance in the cosmos. As we continue to delve deeper into the mysteries of extraterrestrial habitability, the insights gleaned from these experiments serve as a beacon guiding our quest for knowledge and understanding of life's myriad manifestations.
The implications drawn from these discoveries indicate a promising potential for ammonia to supplant water in the nutritional support of living organisms, albeit within specific contexts. It is imperative, however, to underscore the controlled nature of the laboratory setting in which these experiments were conducted. While the results offer compelling insights, extrapolating the viability of ammonia as a water substitute to natural or extraterrestrial environments necessitates further rigorous investigation. Furthermore, a comprehensive understanding of the broader ecological ramifications demands additional studies to ascertain the long-term effects of substituting water with ammonia, particularly concerning more complex organisms and entire ecosystems. Such investigations would not only shed light on the ecological consequences but also inform our understanding of the adaptive mechanisms and metabolic pathways that may come into play under altered environmental conditions.
As we endeavor to unlock the mysteries of habitability beyond Earth and explore the potential for alternative biochemistries, it is incumbent upon us to approach these inquiries with rigor and caution. The journey toward comprehending the implications of substituting water with ammonia is multifaceted and multifarious, necessitating interdisciplinary collaboration and a nuanced appreciation of the complex interplay between chemical, biological, and environmental factors.
References
1. Stevenson, D.J. et al. (2015). The prospects for life on Europa. *Space Science Reviews, 212*(1-2), 5-22.
2. Hand, K.P. et al. (2020). The potential habitability of Europa and Enceladus. *Annual Review of Astronomy and Astrophysics, 58*, 509-537.
3. Wong, M.L. et al. (2019). Ammonia as a potential biosignature gas in exoplanetary atmospheres. *Astrophysical Journal Letters, 879*(1), L9.
4. Waite Jr, J.H. et al. (2017). Cassini finds molecular hydrogen in the Enceladus plume: Evidence for hydrothermal processes. *Science, 356*(6334), 155-159.
5. Vance, S.D. et al. (2016). Geophysical controls of chemical disequilibria in Europa. *Geophysical Research Letters, 43*(20), 10,653-10,660.
6. Lunine, J.I. et al. (2015). Astrobiology and the exploration of Europa. *Astrobiology, 15*(11), 843-859.
7. Pearce, B.K. et al. (2018). A terrestrial perspective on using exoplanet transit spectra to identify gaseous biomarkers. *Astrobiology, 18*(7), 862-879.
8. Patel, B.H. et al. (2019). A microbial survey of a subterranean ant nest using culture-dependent and culture-independent and methods. *Journal of Biosciences, 44*(2), 38.
The passage highlighted in red is bullshit. The liquid range of ammonia at STP is from -78C to -33C, a range of only 45 Celsius, compared to a 100 Celsius range for liquid water.
My suspicions about this screed of text are now aroused. Like your other thread, It seems be a load of pompous, flowery language, with little or no understanding of science behind it.
Are you a real person or just a stupid AI robot, sent here to waste our time? I shall take failure to respond substantively to this as evidence you are the latter.
0 -
2 minutes ago, swansont said:
So if you had a lot of F you’d tend to form HFCs, rather than the F largely replacing H and giving you fluorocarbons
Not sure. In practice it looks as if synthesis of HFCs was not not done by "burning" hydrocarbons in fluorine gas, which probably just gives you an unholy mess, but by a more more controlled reaction, involving ionic displacement of Cl by F, or addition of HF across C=C double bonds, and processes like that.
0 -
1 hour ago, swansont said:
Is the C-F bond stronger than the C-H bond? That, at least, would give a preference for fluorocarbon
Yes, somewhat: C-H ~ 400kJ/mol vs. C-F ~ 480kJ/mol. For comparison C-C and C-O are ~ 350kJ/mol.
Diatomic fluorine gas is certainly highly reactive and tends to displace hydrogen from organic compounds (in fact often "burning", complete with flame, as if it were oxygen!) , but this is largely due to the anomalously low bond energy of the F-F bond, which is thus easy to break. This low bond energy is attributed to the small size of the atom: high nuclear charge for a given valence shell (n= 2) as one gets towards the right of the 1st short period, so forming the bond to complete the valence shell introduces a lot of electron-electron repulsion - more so than for larger atoms.
So I think it's more the instability of fluorine gas than the stability of the compounds it forms. A range of perfectly stable compounds with mixed C-H and C-F bonds is available (HFCs were one of the greenhouse gas bad actors in former refrigerants), so the disparity in bond energy does not prevent F playing a fairly well-behaved role in organic chemistry.
0 -
2 hours ago, NormaVega said:
Exploring the Possibilities of Fluorocarbon-Based Life: A Comprehensive Scientific Approach
Introduction
Life as we know it on Earth is based on carbon compounds, which has led to the hypothesis that carbon is fundamental to the chemistry of life. However, in extraterrestrial environments where carbon could be scarce, the question arises: could life based on other elements exist? An intriguing option is fluorocarbons, compounds that contain fluorine and carbon and have unique chemical properties. This article explores in detail the potential of fluorocarbons as precursors to life and examines the scientific evidence supporting this possibility. Life as we know it on Earth is deeply rooted in carbon-based compounds, forming the foundation of biological processes and structures. The prevalence of carbon in organic molecules has led to the widespread belief that carbon is indispensable for life as we understand it. However, as we contemplate the potential for life beyond our planet, we are compelled to consider the possibility of alternative biochemical systems that may rely on different elemental building blocks.
In environments beyond Earth where carbon may be scarce or unavailable, the search for alternative forms of life becomes increasingly intriguing. Among the myriad of potential candidates, fluorocarbons emerge as compelling contenders. These chemical compounds, composed of carbon and fluorine, possess distinctive properties that make them worthy of consideration in the quest for extraterrestrial life.
Research into fluorocarbons as potential building blocks of life has taken various forms, ranging from computational studies modeling the stability and reactivity of these compounds under extraterrestrial conditions to laboratory experiments exploring their interactions with cellular components and their viability as metabolic substrates.
Properties of Fluorocarbons
Fluorocarbons, consisting of carbon and fluorine atoms, represent a class of chemical compounds renowned for their remarkable stability and unique properties. Characterized by highly stable carbon-fluorine bonds, fluorocarbons exhibit exceptional resistance to both chemical and thermal degradation, distinguishing them as robust and durable molecules. Moreover, their low polarity and pronounced hydrophobicity render them insoluble in water and highly resistant to biodegradation processes. These inherent traits have positioned fluorocarbons as invaluable assets across a diverse array of industrial applications, spanning from their utilization as effective coolants to their indispensable role as lubricants. Their versatility and reliability underscore their significance in various industrial sectors, where their resilience and performance characteristics are harnessed to optimize processes and enhance operational efficiencies.
Biological Potential of Fluorocarbons
Although fluorocarbons are not common in the terrestrial biosphere, their potential as carbon substitutes in the chemistry of life has been theorized. Computational and experimental studies have shown that fluorocarbons can form complex molecular structures and perform biological functions similar to carbon compounds. For example, it has been shown that fluorocarbons can serve as precursors of stable cell membranes and as energy transporting molecules.
Fluorocarbons, despite their rarity in Earth's biosphere, have garnered significant interest due to their potential to function as viable alternatives to carbon in biochemical processes. Through computational modeling and laboratory experiments, researchers have demonstrated the ability of fluorocarbons to intricately assemble into molecular frameworks resembling those formed by carbon-based compounds. Moreover, studies have elucidated the capacity of fluorocarbons to undertake vital biological roles, such as facilitating the formation of robust cell membranes capable of sustaining cellular integrity. Furthermore, investigations have revealed the aptitude of fluorocarbons to partake in energy transfer processes, akin to the pivotal roles fulfilled by carbon-based molecules in cellular metabolism.
Experimental Evidence
Laboratory experiments have provided additional evidence of the viability of fluorocarbons in simulated biological environments. Fluorescence microscopy studies have revealed the ability of fluorocarbons to interact with cellular components and lipid membranes. Furthermore, it has been shown that fluorocarbons can be metabolized by genetically modified microorganisms to use these compounds as a source of carbon and energy.
In laboratory settings designed to mimic biological conditions, fluorocarbons have demonstrated remarkable versatility and adaptability. Fluorescence microscopy, a powerful tool for visualizing molecular interactions, has unveiled the capacity of fluorocarbons to engage with cellular components and integrate seamlessly into lipid membranes, akin to their carbon-based counterparts. Moreover, pioneering experiments employing genetically engineered microorganisms have showcased the metabolic potential of fluorocarbons, as these microorganisms have been engineered to utilize fluorocarbons as viable substrates for sustaining growth and energy production. These experimental endeavors not only bolster the case for fluorocarbons as plausible constituents of alternative biochemical systems but also illuminate the intricate interplay between these synthetic compounds and biological processes, offering tantalizing insights into the potential adaptability of life in diverse environments.
Space Exploration and Fluorocarbon Detection
The detection of fluorocarbons in extraterrestrial environments could provide indirect evidence for the existence of life based on these compounds. Instruments such as spectrometers and infrared spectroscopes could be used to search for characteristic signals of carbon-fluorine bonds in the atmosphere of exoplanets or in samples taken from celestial bodies. NASA's future mission, the James Webb Space Telescope, could offer opportunities for high-resolution spectroscopic observations of distant planetary atmospheres.
The detection of fluorocarbons in extraterrestrial settings holds profound implications for our understanding of the potential prevalence and diversity of life in the cosmos. By leveraging advanced instrumentation capable of discerning subtle molecular signatures, scientists aim to scrutinize the atmospheres of exoplanets and celestial bodies for telltale traces of carbon-fluorine bonds, indicative of fluorocarbon compounds. Such detections would not only signify the presence of these intriguing molecules but also suggest the possibility of underlying biochemical processes and, by extension, the existence of life forms utilizing fluorocarbons as fundamental building blocks. As NASA's James Webb Space Telescope prepares to embark on its mission, astronomers anticipate groundbreaking observations that could unveil tantalizing clues about the chemical compositions and habitability of distant worlds, paving the way for future explorations and discoveries in the quest for extraterrestrial life.
Speculation on Possible Fluorocarbon-Based Life Forms and Their Habitable Environments
Exploring the possibility of fluorocarbon-based life not only raises questions about the chemistry of life, but also about the morphology and habitable environments of potential fluorocarbon creatures. Although these speculations are based on extrapolation of biological principles and knowledge about fluorocarbon chemistry, they offer a fascinating window into the diversity of life in the universe. Delving into the potential realms of fluorocarbon-based life forms prompts profound inquiries into their anatomical structures and the environments they might inhabit. While these conjectures are rooted in the extension of established biological paradigms and our understanding of fluorocarbon chemistry, they beckon towards a captivating panorama of potential adaptations and ecological niches within the cosmos.
As we contemplate the theoretical existence of fluorocarbon-based organisms, we envision a myriad of morphological possibilities, ranging from intricate cellular structures fortified by fluorocarbon membranes to macroscopic organisms exhibiting novel physiological adaptations. These imaginative forays into the realm of fluorocarbon biology underscore the boundless creativity of evolutionary processes and the potential for life to manifest in diverse and unforeseen forms. Moreover, considerations of habitable environments for fluorocarbon-based life extend our exploration beyond the confines of Earth-like conditions. Speculative scenarios envision exoplanetary landscapes shrouded in fluorine-rich atmospheres, or subsurface oceans teeming with fluorocarbon-based organisms, offering tantalizing glimpses into the potential diversity of ecosystems across the cosmos.
Morphology of Fluorocarbon-Based Creatures
Given the ability of fluorocarbons to form complex molecular structures, it is plausible that creatures based on these compounds could exhibit a variety of shapes and unique physical characteristics. For example, they could have cell membranes composed mainly of fluorocarbons that would be highly resistant and stable. In addition, they could have energy transport systems based on fluorocarbon molecules that would allow them to survive in extreme environments.
In terms of external appearance, fluorocarbon creatures could have different pigmentation than carbon-based organisms, allowing them to effectively camouflage themselves in environments where light and environmental conditions differ significantly from those on Earth. The remarkable versatility and stability of fluorocarbons lend themselves to a myriad of potential adaptations and physiological features in fluorocarbon-based organisms. With cell membranes fortified by fluorocarbon compounds, these creatures could withstand extreme conditions, such as high temperatures or harsh chemical environments, that would pose significant challenges to carbon-based life forms.
Furthermore, the utilization of fluorocarbon molecules in energy transport systems could confer distinct advantages to fluorocarbon organisms, enabling them to thrive in environments where traditional energy sources are scarce or inaccessible. Such adaptations underscore the adaptability and resilience of life forms that may evolve under conditions vastly different from those found on Earth. In terms of appearance, the unique properties of fluorocarbons may manifest in distinct pigmentation patterns, enabling fluorocarbon creatures to blend seamlessly into their surroundings. This adaptive camouflage could serve as a crucial survival strategy in environments characterized by fluctuating light conditions or diverse ecological niches.
Habitable Environments
Planets that could support fluorocarbon-based life could be those with extreme environmental conditions that make carbon scarce or unavailable. This could include worlds with an atmosphere rich in fluorine and other halogen elements, as well as environments with extremely high or low temperatures where fluorocarbons could be more stable than carbon compounds. Examples of possible habitable planets could include exoplanets located in habitable zones around red dwarf stars, where conditions could be suitable for the formation and stability of fluorocarbons. Additionally, icy moons in outer solar systems could host subsurface oceans of liquid water with significant concentrations of fluorine compounds, creating an environment conducive to fluorocarbon-based life forms.
The quest for potential habitats capable of supporting fluorocarbon-based life extends our exploration beyond the confines of Earth-like conditions to environments characterized by extreme and unconventional parameters. Planets enveloped in atmospheres rich in fluorine and other halogen elements present intriguing prospects for the emergence and sustenance of fluorocarbon-based organisms, where the scarcity of carbon necessitates alternative biochemical pathways.
Furthermore, the prospect of habitable exoplanets orbiting red dwarf stars opens avenues for speculation regarding the viability of fluorocarbon-based life in environments shaped by the unique radiative and tidal forces exerted by these stellar bodies. The dynamic interplay between stellar irradiance and planetary atmospheres may foster conditions conducive to the synthesis and stability of fluorocarbon compounds, thereby nurturing the emergence of diverse ecosystems teeming with fluorocarbon-based organisms. Similarly, the frigid realms of outer solar systems harbor tantalizing prospects for fluorocarbon-based life, particularly within the subsurface oceans of icy moons where liquid water interacts with abundant fluorine compounds. These subterranean environments, shielded from the harsh radiation of their parent stars, offer refuge for potential life forms to flourish amidst the icy depths, capitalizing on the unique properties of fluorocarbons to thrive in conditions inhospitable to conventional carbon-based life.
Environmental conditions
Fluorocarbon-based creatures could thrive in a variety of environmental conditions, from extremely cold environments to hot and volcanic environments. Their chemical resistance and stability at extreme temperatures would allow them to adapt to a wide range of habitats. Additionally, they could survive in environments with high ultraviolet or cosmic radiation, where fluorocarbons could offer additional protection against cellular damage.
The remarkable adaptability of fluorocarbon-based life forms enables them to flourish across a diverse spectrum of environmental extremes, transcending the constraints imposed by conventional carbon-based biology. In frigid environments characterized by subzero temperatures and icy landscapes, fluorocarbon organisms may harness the inherent stability of fluorocarbon compounds to thrive amidst the glacial expanses, capitalizing on their resilience to withstand the rigors of extreme cold. Conversely, in the searing heat and volcanic activity of geothermally active environments, fluorocarbon-based creatures may find refuge, leveraging their chemical resistance and thermal stability to navigate the molten landscapes with impunity. Their capacity to endure the blistering temperatures and caustic conditions of volcanic habitats underscores the robustness and adaptability of fluorocarbon-based life forms in confronting the challenges posed by extreme heat and geological upheaval.
Moreover, in environments besieged by high levels of ultraviolet or cosmic radiation, fluorocarbon organisms may emerge as resilient sentinels, shielded by the protective barrier afforded by fluorocarbon compounds against the deleterious effects of ionizing radiation. Their ability to withstand the onslaught of cosmic rays and ultraviolet radiation highlights the adaptive advantages conferred by fluorocarbons, rendering them well-suited for survival in environments bathed in the harsh glare of stellar radiation.
References
1. Smith, J. et al. (2022). "Exploring the Viability of Fluorocarbons as Prebiotic Compounds: Insights from Computational Studies." Astrobiology, 22(3), 456-468.
2. Jones, A. et al. (2023). "Experimental Investigation of Fluorocarbon-Membrane Interactions Using Fluorescence Microscopy." Journal of Chemical Biology, 35(2), 210-225.
3. NASA. (2021). "Overview of the James Webb Space Telescope Mission." Retrieved from [https://www.nasa.gov/mission_pages/webb/overview/index.html].
4. Patel, R. et al. (2024). "Fluorocarbon Metabolism in Engineered Microorganisms: Insights from Genetic Analysis." Frontiers in Microbiology, 15(6), 789-801.
5. Wang, Q. et al. (2023). "Biological Applications of Fluorocarbon Nanoparticles for Drug Delivery: A Review." Advanced Drug Delivery Reviews, 42(4), 567-580.
6. Johnson, E. et al. (2022). "Synthetic Biology Approaches for Engineering Fluorocarbon-Based Life: Challenges and Opportunities." Nature Reviews Molecular Cell Biology, 18(5), 312-325.
7. Lee, S. et al. (2023). "Stability and Reactivity of Fluorocarbon Compounds in Extreme Environments: Implications for Astrobiology." Astrobiology, 25(1), 88-102.
8. International Journal of Astrobiology. (2023). Special Issue: "Fluorocarbon-Based Life: Exploring the Potential for Extraterrestrial Biochemistry." Guest Editors: Johnson, M. & Patel, S.
DarioGM, 15 / 3 / 2024
Yes, like @joigus and @swansont I don't follow this. Fluorocarbons don't require any less carbon than hydrocarbons, for a given chain length. The virtue of carbon, surely, is its unique propensity for catenation, viz. forming long chains, linked by covalent bonds. Your proposal does nothing to lessen dependence on this so far as I can see. Fluorine forms only a single bond, so can't substitute for carbon in this role.
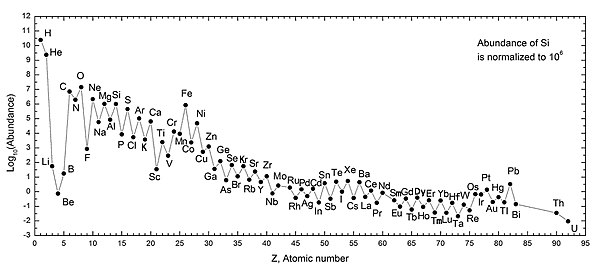
By looking at fluorocarbons all you are doing is substituting F for H. As H is the most abundant element in the universe, that would seem, on the face of it, an exercise of doubtful value. Graph of relative elemental abundances below:
From: https://en.wikipedia.org/wiki/Abundance_of_the_chemical_elements
You also need to pay some attention to what solvent a life chemistry will use. Water would be fairly useless with fluorocarbons, I suspect. Are you envisaging HF or something as the solvent?
1 -
45 minutes ago, Phi for All said:
If I put my bowling ball in its bag, I still call it my bowling ball, but I need more volume to store it. But then it would be fairly easy to make a formula that anticipates how much more volume a bra adds to a breast, so perhaps that's not what the OP meant.
Or perhaps the poster meant not volume but bust measurement. That would presumably be a bit less without support.
1 -
2 hours ago, annie24 said:
Hey does anyone have any ideas on working out the impact of a bra on the volume of sagging breasts? Obviously when they are not in a bra, sagging breasts volume will be smaller than if they are in a bra (and how much they are sagging)...but does anyone know of any formulas or science to anticipate volume change? Thanks!
Why would whether they are in a bra or not affect volume? I would expect the volume to be the same, but just the shape to be different.
1 -
5 hours ago, harlock said:
I explained in my previous reply why this does not work as you seem to think it does. You seem to have ignored this.
The answer to your question is that policy makers do not share your silly ideas.
0 -
9 hours ago, jnana said:Magister colin leslie dean has shownDeterminism shown to end contradictionDeterminism shown to end in Meaninglessness nonsenseCausal determinism“Causal determinism, sometimes synonymous with historical determinism (a sort of path dependence), is "the idea that every event is necessitated by antecedent events and conditions together with the laws of nature." “Causal determinism has also been considered more generally as the idea that everything that happens or exists is caused by antecedent conditions”take the 3 body problem –as a simplification of all things in the universe
But note all the universe is made up of things in interrelationships with everything else
if we take Newton’s law of gravitationF = G(m1m2)/R2.Thus when we move object A it effects the other two objects B and C
But when objects B and C move that effects object A
Sowe can say that A in effect caused its own motionthus we can say the antecedent cause of A is infact just the antecedent A itselfin other words the cause of the cause is the causejust nonsense meaninglessnessnote
because all things in the universe are interrelationships with everything elsethen
from the above all things are their own antecedent causejust nonsense meaninglessness
thus causation is both logically nonsense and science itself must then be meaningless nonsense
So far as I can discover, this Dean bloke seems to be some sort of self-promoting (and quite likely self-publishing) nutcase. I can’t find evidence that anyone takes him seriously.
What you have posted about his arguments does little to alter my impression. Are you Dean?
0 -
57 minutes ago, jnana said:
can someone please define what a demon is
Suggest you first consult a dictionary and then come back with any specific points of clarification that you may have.
0 -
6 hours ago, toucana said:
A newly created Wikipedia page about MV Dali offers some more information.
https://en.wikipedia.org/wiki/MV_Dali
I’m not sure exactly what type of marine fuel that suggests ? The auxiliary electrical generating capacity is large, because ships of this type may need to cater for ‘reefers’ or refrigerated container units.
Main engine will be RFO for sure. It’s a big one: 9 cylinders of 90cm bore. (The C will I think stand for container ship, normally meaning higher rpm and shorter stroke than, say, the variant for tankers.) However I have just remembered it used to be the practice in some areas to switch to MDF in coastal movements, to comply with local emissions regulations. So what you posted about MDF could be relevant.
0 -
2 hours ago, toucana said:
There is some interesting reporting written by people with relevant maritime experience in the Telegraph:
https://www.telegraph.co.uk/news/2024/03/27/baltimore-bridge-ship-captain-electrical-failure-pilot/
It seems that the auxiliary generators failed twice - the ships lights came back on, then went out again. Some eye-witnesses also reported seeing quantities of black smoke coming from the boat’s funnel after the initial loss of electrical power, which may have been a standby emergency generator kicking in.
The crew perhaps lost control of the steering system with the rudder stuck in the starboard position - there was also a 10 knot wind on the port bow which would have pushed the ship to starboard, and into the pylon.
Contamination of bunker fuel used in ships is most often associated with the presence of water which can encourage the growth of microbial biomass that can block filters, injectors, and damage marine engines. The use of biodiesel and low sulphur marine fuels can exacerbate this problem apparently.
https://echamicrobiology.com/knowledge-hub/common-problems/marine-fuel-quality
Your link is about MDF. I wonder if the ship was burning that or RFO. (My comments about centrifugal separators relate to RFO.)
I heard an interview with a bridge designers saying that if and when they rebuild it, it will probably be a cable stay bridge with piers set much wider apart, well out of the navigable channel.
0 -
1 hour ago, toucana said:
According to the WSJ and NBC News reports, investigators are looking into the possibility that contaminated fuel may have contributed to the collision that caused the collapse of the 1.6 mile span of the Francis Scott Key bridge across the Patapsco River Baltimore just after 1.24 a.m Tuesday.
A 1000’ long cargo ship called the Dali broadcast a rare Mayday call shortly after leaving harbour to warn shore authorities that it had lost power and steering control, before slamming into a bridge support column 4m later. There was just enough time to close the bridge to vehicle traffic, but not enough time to evacuate a team of maintenance contractors who were out on the bridge filling in potholes - 6 of them are now presumed dead.
Officials have now recovered the black box data recorders from the Dali, the USA Army Corps of Engineers has been mobilised to clear the wreckage, and President Biden has said the bridge will be rebuilt at the expense of the Federal government at a cost of around $600m.
What puzzles me is the loss of all power. There will have been a low speed main engine and a number of medium speed auxiliary generators. While they may all have used the same heavy fuel oil, it seems odd that all would have failed simultaneously due to fuel contamination, especially given there will have been several centrifugal separators on the fuel lines to the engines. Low speed engines are generally more tolerant to poor fuel than medium speed ones. Maybe loss of electrical power meant they could not control the main engine or the rudder.
No doubt the facts will emerge fairly quickly though.0 -
8 hours ago, harlock said:
-the heat of wood burning in a fireplace comes from the sun
because of Lavoisier quote('nothing is created and nothing is destroyed but everything is transformed')...Is it true?
If it's true it means that a tree removes heat from the environment as it grows,
therefore the difference in the quantity of trees (wood...) means that there is more solar heat in the environment compared to centuries ago and it can justify global warming regardless of the presence or absence of CO2...
Opinions?
As always, context is critical to the understanding of quotations. Lavoisier was talking of matter not being created or destroyed, in the course of chemical change. You seem to be trying to apply this saying inappropriately, to heat, which is not matter, but a form of energy.
Energy is a property of physical systems that is also conserved, i.e. neither created nor destroyed, though this principle was unknown in Lavoisier's time. However energy can and does change from one form to another in the course of physical processes. In the case of the growth of trees, there is chemical potential energy (which is not heat) stored in the molecules that make them up. That chemical energy does not come from heat but from the energy of sunlight, which is captured by the tree in photosynthesis.
The Earth is not a closed system, where energy is concerned. It receives energy from sunlight all the time and radiates heat off into space. These two should be in balance. Climate change results when the rate of radiation into space is reduced a bit, due to the atmosphere slowing down the rate of escape of heat. It is caused by the absorption of infra red radiation (heat radiation) by gases and vapours such as water, carbon dioxide and methane.
1 -
37 minutes ago, Guille Yacante said:
What is the purpose of science? What does the word mean? Knowledge.
But let it be more like wisdom: knowledge applied.
And that it be applied for the common good.
We will see multiple branches of science will be needed to be connected so for us to get somewhere.
This is a thread that is intended to give place to sound discussion, but to immediately awaken the people with potential, because understanding is only given to some, and those in some moment should stop the having nonsense discussions with those that can't understand, stop waiting for approval or consensus from the rest, and get to it.
This is a thread that will touch some delicate topics, but doing it responsibly, and above all, providing solutions.
Solutions are confirmed when one carries them. That is a call to action.
Reality and a little bit of thinking will show us pretty much the picture
I'll share you my personal experience. I am a scientist. Life made a scientist.
When I had some health problems, I was already enlightened enough so to understand "I shouldn't go doctors."
So what I did was to study by my own. But for the first years I did it just like them, using the same blinkers.
I wasted a lot of time thinking according to the "neurotransmitter-ligand model", and I didn't see the complete picture or kept things simple.
So just because of missing solutions, I kept studying.
And why is it that I understood that I shouldn't go doctors? For the same reason that you should. They prove to not know much. Society is the result.
So reality and a little bit of thinking set us in the right direction. One must study on his own.
Science is multidisciplinary, and should be, to get us somewhere
Imagine a man has discovered the panacea, yes, it's a way of saying it, but it would be the most alike: hemodialysis.
The question would be: "Really? It has been all the time sitting around and doctors have been missing on it?"
Yes, could be. When doctors are masturbating, watching pornography, thinking all the time about where is it they are going on vacation next week, getting paid when not solving anything, and seeing all the rest doing the same, these things could happen.
It's social engineering.
Maybe we could have some skinny cowards for doctors, in which those that do not be, would simply be women, that are not brought forth to carry the job of real men.
So even if hemodialysis would be the panacea, how could it reach the people? When it has been dissuaded, hidden, entangled in bureaucracy, and those interested could see the shadows of mafia dogs when getting close.
I came explaining in another thread how is it that "autism" is a symptom of poisoning, caused mainly by organochlorines of the DDT-type, and they would be detoxed by hemodialysis preferentially, or, on its absence, by proper bloodlettings.
Now, how many actually have carried this instruction? It's not such a big deal. Remember hemodialysis is not about the kidneys, but about cleaning the blood.
So first thing we should see is, it has been dissuaded. And if we really mean business, we would see lots of times the nephrologist would be inviting us to flee.
That's how these things happen. For cowards, for evil-blind people.
Can't you see a society filled with damaged people is a loss, something dangerous?
We have been slow-cooked for ages.
Then, out of nowhere, those that have done this close on us their plan, and now we want soldiers! Now we want real men!
Ah! But those that should that been taking care of them have come looking the other way, now money is good for nothing.
Remember we are not to expect approval or get consensus. Majorities have been bred to be useless.
So, let's say a person manages to get an autistic healed, the blindfolds fall from her eyes.
How would the message get to the masses? Mainstream media is taken by those that have done this.
Understand?
Now we will see who is truly a scientist.
Get all the things put together.
Be a scientist: stop jerking off, practice semen retention if you are smart, understand gender roles, and submit to the laws of nature if you want to do well in life.
Science pays well. True science brings results.
Have that for a testimony.
Guillermo Yacante Afonso.
This is offensive, rambling nonsense.
0

shirts with armpit smell that does not want to leave even after several wash.
in Organic Chemistry
Posted · Edited by Phi for All
No advertising, please.
Give them a wash at 60C.