-
Posts
1059 -
Joined
-
Last visited
-
Days Won
8
Content Type
Profiles
Forums
Events
Posts posted by sethoflagos
-
-
17 hours ago, exchemist said:
Well then, since the spin of ¹⁴N is +1, that of ortho N2 will be +2, with potential components +2, +1, 0 -1, -2, so 5-fold degeneracy, vs single states for the para version. I suppose this should show up in entropy calculations for nitrogen in some way.
I'm not aware of there being such a marked anomaly in nitrogen specific heats as for hydrogen. Perhaps it's something to do with the relative atomic masses. Without knowing the dU and transition temperature values, it's hard to evaluate. But it should appear much like a gradual phase change over some specific temperature band. My guess is that the effect is rather small otherwise it would be cropping up in the literature quite frequently.
0 -
35 minutes ago, Ken Fabian said:
I am not so convinced the confidence in science linking fossil fuel burning to significant and harmful climate change went so far back as the 1930's. Most of the significant factors affecting climate stability were recognised and to some extent quantified but it was a long way short of what was needed for confident prediction; in some respects it was remarkable that a warming "signal" could be found at all, eg by Callendar.
There are (as usual) many good points in what you say. But I'll counter with a question.
Why when meteorology was quite a long established discipline, did it require a steam engineer, Guy Callendar, to ring the alarm bells?
The key is I think summed up in this quote from https://www.thermopedia.com/content/796/.
QuoteFurnace Radiation
In most process heaters, the major part of the heat transfer from the hot gases to the tubes is by radiation. To calculate the radiative component it is necessary to know the effective emissivity, εg, of the combustion gases (typical value 0.25). This is dependent on the ratio of the partial pressures of CO2 and H2, the temperatures of the gas and the radiation source and the effective size of the radiating gas cloud. The latter is represented by the term pLo, the product of partial pressure and effective length of the furnace—a term first introduced by H. C. Hottel. For details of the procedure see Hottel and Sarofim (1967) and Hewitt, Shires and Bott (1994).
The rate of heat transfer to the furnace product is also a function of the geometry of the tube banks and the fraction of the furnace surface area covered by them.
In other words, in order to design an efficient furnace, one needs a very clear understanding of the emissivity of CO2. A level of understanding that wouldn't be at all common in other disciplines (such as meteorology).
Combustion engineering was a fairly mature technology by the 1930's and it would not have required a genius among them to extrapolate the behaviour of CO2 inside the furnace out into the wider environment. Indeed, various individuals had been doing this over the previous century or so.
Callendar was simply the first to collect a reasonable dataset of historic climate records that indicated increasing anthropogenic warming correlating with an understanding that was largely concentrated within the energy and utilities sectors of industry.
I guess that some meterologists at the time might have had there noses put out of joint at being upstaged by a mere engineer.
0 -
17 minutes ago, John Cuthber said:
And you picked "beige" from all of them.

Sometimes I just can't hide my natural flair for colour coordination 😋
0 -
1 hour ago, zapatos said:
And I'm just saying you don't have to go to a restaurant to do so. Restaurants don't have exclusive rights to the ingredients and recipes that go into their cultural foods.
Try cooking something new at home once in a while. It's not magic.
Actually, I enjoy cooking for myself very much and aren't too bad at it. Here's one I did earlier:
I'd caught the yellowfin that morning. The sauerkraut, lime pickle and pickled onions are all homemade from local produce, other than the spices. And the darjeeling of course.
The big plus of preparing your own food is that you can make whatever you want regardless of anyone else's styles and tastes. This one's a real mish-mash of different traditions so you'd be hard pressed to find anything like it on a regular menu.
But you do need to dip into other styles and traditions sometimes whether to extend your repertoire, or just for the simple enjoyment of it.
0 -
7 minutes ago, exchemist said:
I suppose there are also in theory spin isomers of other diatomic gases. But probably the spacing between rotational levels is too small for this to produce discernable effects. It’s something I’d never previously thought about.
Check entropy of mixing. The slightest difference in particle properties produces a significant step change in entropy irrespective of the degree of difference. It's this aspect that got me thinking about these spin isomers in the first place. Again: Gibbs' Paradox.
0 -
17 minutes ago, zapatos said:
I really am spoiled when it comes to meal time. My wife searches out and tries new and different recipes on a weekly basis, and is comfortable making up recipes herself. She also insists on 'getting it right'. A year or two ago she decided her caramel-pecan sticky rolls could be improved. She made them probably twice a week for about two months till she got the dough/caramel/etc. just right. Of course I spent the two months after that getting my pants to again fit just right... 😁
Hey, I'm not knocking your domestic arrangements.
But we are all born into cultures that have a relatively restricted diet. Here in Nigeria I have been able to expand that diet to include such delicacies as Ethiopian nightshade, pumpkin leaf and pan-fried locust. I'm not particularly recommending these to anyone else out there, but I do think it's important to understand for instance that insects can be a viable (and surprisingly tasty) food supplement. I'm not particularly keen on the various preparations of cassava, but yam is a perfect starchy component of stews. I'm just saying that we all have a certain tunnel-vision regarding food that is culturally ingrained. We can all benefit by expanding our cultural horizons to make us less dependent on the specific diets we grew up with and along the way maybe find some unexpected delights.
0 -
14 minutes ago, Sensei said:
Unsure. You tell me:
Too late chum. Your comment was entirely inappropriate so welcome to my blocked list. You've nothing to say worth hearing.
0 -
10 minutes ago, Sensei said:
..westerners are truly depraved by wealth..
That wouldn't be directed at me, would it?
0 -
11 hours ago, zapatos said:
I have the exact opposite view. My wife likes to cook and is very good at it. I just told her the other night that 'if you can cook well, there is no reason to go to a restaurant for good food.' Other than at high end restaurants, my wife normally out-cooks restaurant 'chefs'.
Not everyone likes to cook and is good at it (like me), so if I had to cook for myself all the time the quality of my meals would go down quite a bit and I'd be looking more fondly at restaurants.
Interestingly I now find that restaurant food has lost almost all appeal to me.
I beg to differ somewhat (though I do understand where you're coming from)
One thing a good restaurant does offer is the opportunity to try something new and be educated in a relatively safe environment.
Yes, they can be a bit of an expense, but one expensive treat a year isn't going to break the bank. I try to make a habit of giving myself at least one treat a year, just to make what I do seem somehow worthwhile.
Anyway, my treat for 2006 (I think) was to take my mother and children for week's holiday in Montparnasse. The deal was they could go wherever they wanted during the day, but the evening meal was a proper sit down job somewhere decent (ie my choice!).
The highlight came at La Coupole (I think). I just remember the expression on my mother's face looking first at her 'adventurous' choice of tartare de dourade and then at my plate of la grande choucroute. Fortunately for her, I quite like raw fish and was happy to share. It was a magical bonding moment, and not lost on my then teenage son and daughter.
So while I'm quite happy with regular vegetable soup and bacon omelette sandwiches on a day-to-day basis, it's good to know that there are many, many options out there.
0 -
1 minute ago, exchemist said:
Ah the light dawns - maybe. I didn't realise that by "extra degrees of freedom" you might have in mind the 3-fold degeneracy of the triplet rotational states. If so then, er, yes, we're saying the same thing!
Now I can sleep with an untroubled mind!
0 -
51 minutes ago, exchemist said:
Hmm, I see what you mean. There are no extra degrees of freedom, though. Diatomic molecules all have 2 rotational degrees of freedom. But ortho can only populate odd numbered rotational energy levels while para can populate only even levels. I had to look this up (it's badly explained or not explained in Wiki) but it appears the issue is that ortho hydrogen is a triplet state, in which the total nuclear spin of 1 can be orientated +1, 0 or -1 with respect to the axis of rotation, thereby multiplying the numbers of rotational states available by 3, i.e. each rotational level has 3-fold degeneracy, whereas the para states do not. So at RTP, with kT>> ε for rotation, you end up with a 3:1 ratio, just because there are more ways for ortho to have a certain amount of rotational energy.
I think that's it, at least.
I'll have to sleep on that one. When I wake up may be I'll have figured out how what you said was different to what I said. Thanks nevertheless!
0 -
4 hours ago, exchemist said:
For a while at least. Though from what I read, the rotational J=1 state of ortho does eventually relax to J=0 as the ortho gradually converts to para, by the various non-radiative means of relaxation we have been discussing.
I found the discussion of J state populations a bit hard to follow. Is the 75% ortho equilibrium limit simply due to equipartition amongst these extra rotational degrees of freedom, or is there more to it than that?
0 -
1 hour ago, exchemist said:
The problem with trying to tackle it mechanically is that means quantum mechanically.
What has happened is the wave functions of the orbital have become distorted by the nucleus being off-centre. One can't really speak of nice neat forces, acting between the nucleus and electrons as particles, in this scenario. So the energy approach, which is what the Hamiltonian does in Schrödinger's equation, seems to be the only way to describe what happens, so far as I can see.
I'm not sure how the heat capacity stuff relates to what we have been discussing. Can you elucidate?
The mental picture I'm getting is that the electron fields recoil first from the collision due in part to their much lower inertia. The consequent relative shift of -Ve potential away from the point of impact then acts on the nuclei and as @swansont says, drags them along.
In the extreme case, this mechanism fails and the protons and electron seperate leading to a plasma, which we know happens so that's consistent. It's a classical picture, but there's room for a buch of vitual photons to be showering the nuclei to create some sort of Feynman diagram out of it.
As regards the isomeric transition perhaps there's some really narrow window in the collision spectrum where the colliding nuclei are sufficiently closely aligned for an exchange of spin states to occur? The energy transition is in the same ball park as the latent heat of vapourisation (1.445 kJ/mol vs 0.904 kJ/mol) so the kinetics seem credible even if the actual mechanism is opaque.
In passing, I find it intriguing that such a quantum oriented phenomenon can have such a marked impact on bulk thermodynamic properties. The anomalous behaviour of the specific heat of hydrogen at low temperatures has been known for a long time of course. It's a classic case of Gibb's paradox: the tiniest imaginable difference between particle species is sufficient to cause a significant step change in macroscopic entropy.
0 -
40 minutes ago, swansont said:
The electrons attract nucleus. If you move the electron cloud around, it will drag the nucleus with it.
How does this work? It seems to suggest that the electrons further away from the nucleus must exert more attractive force than those closer. Or is it a case of 'more electrons behind than in front'? If so, I'm struggling a little with the inverse square aspect of Coulomb's Law.
0 -
1 hour ago, Ken Fabian said:
Businesses being responsible and accountable for harms done under the law has always been compatible with and even essential to capitalism as an ideology. When environmentalists were the only voices people were hearing on climate it was easier for business lobbies opposed to accountability on behalf of their members to associate the issue with "anti-capitalist" fringe politics; those leaning right have been strongly discouraged from taking up the issue or admitting there is legitimate grounds for regulatory intervention - but that is no longer so clearly the case.
Let's be clear on this. From at least the mid-1930's the energy sector has been fully aware of the long term impact of burning fossil fuels due to the advice given by its chemists and chemical engineers who understood the principles first clearly articulated by Svante Arrhenius in the 1890's.
Their position has been uniformly duplicitous ever since. They have not the slightest interest in rational debate. They see it simply as a war of words. We owe it to our children and grandchildren to respond accordingly.
3 -
1 hour ago, swansont said:
But such transitions could be induced in collisions
Getting back to the OP, how are the momentum and energy transfers in a collision actually transferred to the nucleus?
I can picture electrostatic repulsion and possibly even the Pauli exclusion principle acting on the electrons, but I struggle to picture the corresponding forces of repulsion acting on the positively charged nucleus.
Okay, I admit it, I've not the faintest idea of what keeps a nucleus centralised within the atom.
0 -
12 minutes ago, studiot said:
No apology needed. If it is really inadequate the please do speak up.
But do you realise that ScienceForums offer the full sized scsn by clicking on the pictures ?
In my browser (firefox on and old XP Dell) this opens a new browser window whcih you can zoom to full size.
That's neat! Thanks!
0 -
45 minutes ago, exchemist said:
How interesting. Molecular hydrogen can get adsorbed onto iron compounds and may dissociate on the surface into atoms. If it does that, then I would expect it to prefer to pair up with an atom of opposed spin before desorbing again, since that has the lower of the two energy states. But I'm just guessing about the mechanism.
So you go with electrochemical. Makes sense.
A smidgen more:
Wikipedia tells me that the energy transition from para to ortho is 1.455 kJ/mol. Dividing by Avogadro's number and Planck's constant gives a frequency of 3.646 THz which just about crawls into far infra-red, I think (if I've done it right. Not a calculation I often get asked to do!).
Also, I find it intriguing that it's impossible to obtain by heating an ortho/para ratio that's higher than 3:1. Is it purely coincidental that ortho is a triplet state and para a singlet?
PS : Apologies - @studiot's attachment is beyond the capabilities of my prescription lenses!
0 -
50 minutes ago, exchemist said:
I would imagine it has to be via emission and absorption of microwave radiation corresponding to the energy of the transition between the two states, as in nmr.
Later note: Sorry, no, it can't be. The probability of spontaneous emission at RF frequencies is negligible, because of the ν³ rule. It must be the presence of fluctuating external fields from neighbouring molecules that does it, cf. spin-lattice relaxation in nmr. (This was all a long time ago so my recollection is hazy, to say the least.)
It may be relevant that the transition rate can be sped up by a factor of twenty or so in the presence of an iron catalyst. The immediate assumption would be that some sort of electrochemical mechanism was in play, but perhaps it's more magnetic than chemical.
0 -
One more conspiracy theory to add to the list, then?
0 -
4 hours ago, Matt Wieland said:
looking for a "unique" chemical or maybe a combination of chemicals to make it "unique"
also looking at chemical that could be seen under UV only when exposed to H20...any thoughts?
Looking at the absorption spectrum of liquid water ;
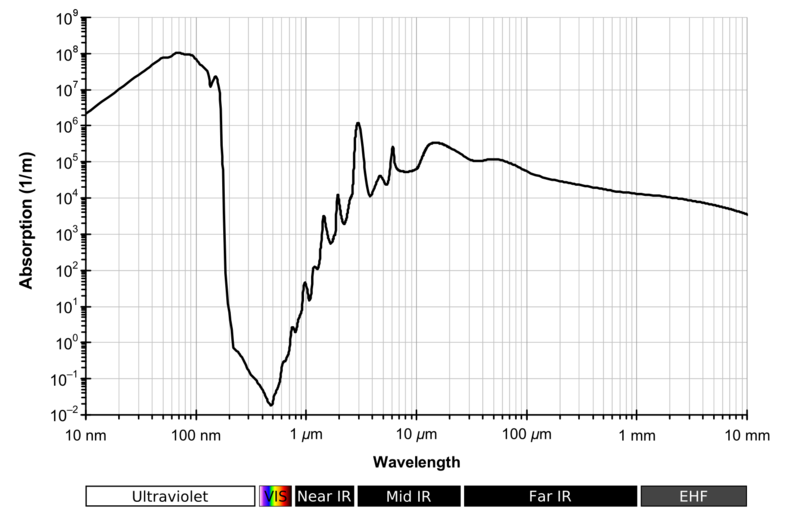
Doesn't this make it essentially black to UV? Seems to defeat the object.
0 -
I was reading up on the equilibrium relationship of ortho and para (molecular) hydrogen (ortho having proton spins in parallel alignment, para ant-parallel).
With para hydrogen being the lower energy state, relatively pure para hydrogen (~99.8%) can be obtained by chilling pure hydrogen down to around 20 K and keeping it there for a couple of days.
If this is rapidly heated to a more moderate temperature and then kept fully insulated, the gas will slowly lose temperature as the the appropriate ortho/para equilibrium is established. Essentially an endothermic reaction. Though it can take several days to do this.
It's sort of a remarkable behaviour in itself but it got me wondering exactly how the protons manage to extract energy from the bulk internal energy of the gas. I'm guessing the surrounding electron orbitals aren't the insulating cushions I'd pictured them to be.
0 -
1 hour ago, Endy0816 said:
If there's one word that sends chills down a chemical engineer's spine it is BLEVE (and it needs to!).
Post script: LPG is often stored in elongated cylinders informally referred to as 'bullets'. A common 'good design practice' is to avoid aligning them with the long axis pointing towards any habitation within 3 kms.
0 -
2 hours ago, Innocent Muggle said:
Hello al!

I wonder how to calculate amount of dew in the morning per unit area.
set the temperature go down 20c from 40c and unit area is 1m square.
I hope some one explain the formula for this!
thank you!
How long have you got?
Firstly, you need to know the water carrying capacity of air at your lowest temperature.
This is calculated via the saturated vapour pressure (Ps1) of water at this temperature (T1) for which the August Roche Magnus equation is probably most convenient and simplest (it derives from the Clausius Clapeyron equation qv)
Ps1 = 610.94e^(17.635(T-273.15)/(T - 30.11))
Where units are Pa and K. For T = 293.15 (20 oC) this gives Ps1 = 2,335 Pa
The ideal gas equation (PV = nRT) then tells us that the maximum molar water vapour capacity per m3.
ns1 / V = Ps1 / RT = 2,335 / (8.3145 x 293.15) = 0.9573 gmol / m3
Taking 18 as the mol wt of water that gives a mass density of 18 x 0.9573 = 17.23 g / m3 @ 20 oC.
That's more than enough for one post.
Tell me if you follow it so far and we can move on to an easy bit followed by a hard bit.
0




What are you listening to right now?
in The Lounge
Posted
Over the last 25 years or so it has been highly amusing to see German heavy rock band Rammstein quietly but consistently appropriating and repurposing right-wing iconography to serve politically progressive purposes. It's a brilliant strategy. Absolutely immune to any accusation of 'wokeness'. But at the cost of being somewhat challenging to those who want nothing to do with that particular kind of iconography.
For those of a nervous disposition, the title means exactly what you think it means so feel free not to view if you think you'll be offended. Though by doing so you'll miss some beautiful wind band intros and outros by members of the Dresdner Staatskapelle Orkester which would be a pity.