

Erik2014
-
Posts
15 -
Joined
-
Last visited
Content Type
Profiles
Forums
Events
Posts posted by Erik2014
-
-
Hello!
Humanity can make up for the lack of energy and at the same time maintain the ecological balance of the native planet. How to do it? The answer is offered on three pages. This is a real chance.
Thank you for your attention.
Little edit, sorry.
0 -
Hello!
Humanity can make up for the lack of energy and at the same time maintain the ecological balance of the native planet. How to do it? The answer is offered on three pages. This is a real chance.
Thank you for your attention.
0 -
First page jpg-format here (CO2engl2_01.jpg).
Or see (all pages pdf-format):
http://www.youblisher.com/p/871437-Energy-source-evaporation-condensation-Editing-on-April-20-2014/
0 -
Editing by March 26, 2014Or see attachment: CO2engl_01.jpg, CO2engl_02.jpg, CO2engl_03.jpg - 1, 2, 3 the pages.
Regards, Emil Kutin
P. S.
Link 12 March invalid.
0 -
Editing 12 March
http://www.youblisher.com/p/838827-Energy-source-evaporation-condensation-Edited-version-2/
Regards, Emil Kutin0 -
I edited the article.
http://www.youblisher.com/p/825780-Energy-source-evaporation-condensation-Edited-version/
Regards, Emil Kutin0 -
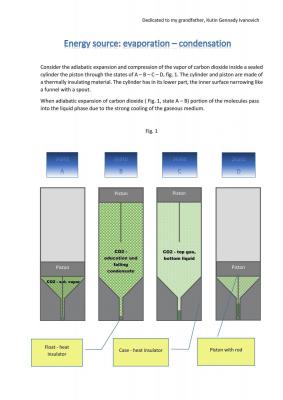
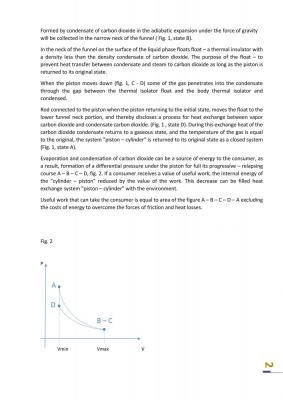
Eyeballing it, your graph seems to be missing a pressure drop between B and C.In the transition from position B to position C (Fig. 1) the CO2 condensate drops down beneath a bobber - thermal insulator. B and C are shown for clarity, improved perception, better understanding. Pressure at passage B to C is not changed for this logical model. The point of this paper is to show that the evaporation and condensation in an adiabatic process of expansion and contraction can be used as a renewable and environmentally friendly source of energy.0
-
You would need to compress and cool the CO2 to cause it to form a liquid. Then to cause it to expand you will need a heat source and obviously decompression.
I mean that CO2 expands adiabatically cooled, thus there is a partial condensation of CO2. Expansion takes place under the pressure of CO2 and CO2 cooling (condensation of CO2) is a consequence of the adiabatic expansion of CO2.
Unfortunately, I did not understand about what you wrote on. But you also do not seem to understand ...
0 -
Where does the energy for the initial pressure come from?
Starting position can provide battery.
R744 really isn't my favorite refrigerant anyways. R12 or R134a would likely serve you better IMO.
Yes, any freon - a matter of taste.
CO2 should be left for dry ice systems, deposit free cleaning and possibly Mars exploration.
As you wish. I do not forbid you.
50 bar is almost ~50x higher pressure than standard pressure.
It's almost pressure that's at 500 meters below sea level in ocean. Water mass is 500 tons per m^2 at such depth.
Submarines from II war were squashed at 200... 280 meters, at twice smaller pressure.
Submarine needed buoyancy in the water. Submarine must be definitely easy to drown. This limits its structural strength.
Installing energy generation based on evaporation and condensation processes can stand on the ground and be quite heavy and solid.
Pressure will be so high, it's unlikely to push piston back...The validity of the principle should be considered in an ideal system. In an ideal system there are no forces of friction and heat losses.
Piston returns to its original position if the work of expansion equal to or greater compression work. The paper shows that the work of expansion more the work compression, see fig.2.
Normal piston is releasing gas that's inside when it'll go to upper est position, then piston goes in reverse direction, because it's empty and nothing disallows going it in that direction. Then there is injected fuel, and spark is igniting it, and changes liquid to gas which pushes piston again. And everything is repeated.
You want to push piston back, without releasing CO2 from inside.
Yes, this is normal.0 -
Under standard pressure, normal temperature (on Earth), CO2 dry ice goes directly from solid state to gaseous state skipping liquid state (sublimation) at -79 C.
So "bottom liquid CO2" from image C, the most probably, will never happen.
Yes, piston would be pushed up.
But why on image D it would go back to "squeezed"?
The initial pressure above atmospheric pressure (50bar, +15C), and therefore, the liquid phase is.
The piston performs a reciprocating motion, the piston may be connected to the flywheel through a curvilinear rod mechanism, not shown in the figure.
I think you could use ocean pressure to condense it. Bit extreme but could be done. More concerning is the lack of mention of an external energy source to evaporate it again.
It is possible to implement a heat exchange with the environment.
Looks like a perpetual motion attempt if I'm understanding it correctly. Could be wrong but what it appears to me as.
You are not mistaken, it should be a perpetual motion machine of the second kind. This engine, which performs the work at the expense of the internal energy the environment or due its self-cooling.
For the most part the paper itself is readable. Still could use proofreading by someone local. Maybe they could help clarify the meaning as well if we are in fact misunderstanding it.
I think that you understand the job right.
0 -
I do not know English, I use online translation, so there is a problem in communication. Article translated by a professional translator and I hope that the article is clear.
0 -
Can you at least post something of an abstract here? Something to whet one's appetite, i.e. to give us a reason to download the document and read it?
It serves hot sauce Maxwell demons. As a result, you get the calories and solution to the paradox. I hope it suits your needs?
0 -
"Could you possibly have a go at summarizing your document in the thread?"
I'm sorry, I did not understand what you want? Please return back my topic.
0 -
New idea.
Energy source: evaporation - condensation.
See the attachment. CO2engl2.pdf
Regards Emil Kutin0


Energy source: evaporation - condensation
in Speculations
Posted
Dear Sirs!
I bring to your attention a solution to a world problem: ecology and energy.
The source of energy for our needs can be evaporation and condensation. How can it be? The answer to this question is on three pages.
Interesting reading!