Leaderboard
Popular Content
Showing content with the highest reputation on 10/21/21 in all areas
-
NIH: Statement on Misinformation about SARS-CoV-2 Origins Unfortunately, in the absence of a definitive answer, misinformation and disinformation are filling the void, which does more harm than good. NIH wants to set the record straight on NIH-supported research to understand naturally occurring bat coronaviruses at the Wuhan Institute of Virology, funded through a subaward from NIH grantee EcoHealth Alliance. Analysis of published genomic data and other documents from the grantee demonstrate that the naturally occurring bat coronaviruses studied under the NIH grant are genetically far distant from SARS-CoV-2 and could not possibly have caused the COVID-19 pandemic. Any claims to the contrary are demonstrably false. Full statement: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-misinformation-about-sars-cov-2-origins2 points
-
So, imagine I'm a professor teaching a class. I refer to students by their preferred first names. Plenty of people go by things different from what's on their birth certificates. Some are kind of odd, but generally I do my best to pronounce people's names the way they ask, and not mix up people's names. I make the odd mistake, but generally that's how it goes. Except for the African American kid. I call him "boy" because that's what we call black folks where I'm from. He repeatedly tells me his name is Paul, but I insist on calling him "boy" whilst using everyone else in the classes preferred name. Paul complains to the University office for the Prevention of Discrimination and Harrassment, who determine that I have violated both institutional policy and federal employment laws by discriminating based on race. I have to face a disciplinary hearing and might get fired. Now replace "name" with "pronoun" and "African American" with "transgender". Explain why it wouldn't be discrimination based on gender identity.2 points
-
Zapatos, poor sod, has never witnessed the passion of earthworms mating and the forbidden love between a toad and a cat, the love that dare not speak its name. From the humble paramecium and its many paramours, to the literate and steamy sonnets of the witty dolphin, the biome is drenched in love!2 points
-
! Moderator Note To all: Please note that the discussion here will not focus on Lazar or his claims. If it helps, simply substitute "unknown stable element" as the target Yes, your spectroscopy would yield an unknown (previously unobserved) spectrum. You could pop a sample into a mass spectrometer and get the atomic mass. You could try to fully ionize it to get the atomic number. If you can make it into a hydrogen-like state (i.e. one electron), the spectroscopy would be easy to predict and compare. There is also Moseley's law, which predicts the x-ray spectrum https://protonstalk.com/physics/moseleys-law/ You could bombard it with some energetic particle (e.g. neutrons or protons) and look at the particles produced.1 point
-
I disagree - that's the EXACT point of Peterson's argument. He doesn't believe in transgenderism (despite the extensive scientific basis of it), and is claiming that being asked to use a student's preferred pronouns is a violation of his free speech rights. His subtle, intentional discrimination is that he would be happy to use the preferred pronouns of cis-gender appearing students, but not those whose physical appearance does not conform to his assumptions of gender presentation, because of his personal (and IMO fundamentally wrong) opinion that their identity is not valid. We're going in circles, but he's arguing that it is his right to discriminate against people who do not conform to gender norms. I'm pointing out that it's not for other protected classes, and asking why transgender people shouldn't be afforded the same protections as religion, race, sexual orientation, etc.1 point
-
I think the answers that you got here are more than enough to go by. But let me try and add a very strong inkling that the quantum must be more fundamental. There is a well-known result of formal (mathematical) quantum mechanics called Ehrenfest's theorem, which tells you that whatever evolving quantum configuration reproduces classical physics at least for the expected values. So classical mechanics is somehow in the guts of quantum mechanics. But there is no way that you can get the quantum with all its peculiarities from classical mechanics. The closest you can get to that is very vague, but it does exist. It's called the Hamilton-Jacobi equation. Some people like to say that, had William Rowan Hamilton spent one more sleepless night, he may have surmised something like quantum mechanics was plausible. But I think that's an overstatement. How would he have figured out the need of a fundamental constant like \( \hbar \)?1 point
-
So does the professor on the podium. Jordan Peterson has been forcefully and very publicly making the point that he shouldn't be required to respect the stated identity of anyone he considers unworthy. When someone has noticeably different different pigmentation, their racial identity is not questioned - though their 'hypersensitivity' is often cited as the reason for complaint, rather than the disrespectful speech itself. But if the intentional discrimination is on the basis of gender identity, which is not outwardly visible, their claim to it can be denied. If the student complains, it's the student who is called delusional, a nutbar, and worse. Who are these extreme activists? What, specifically, have they done and to whom?1 point
-
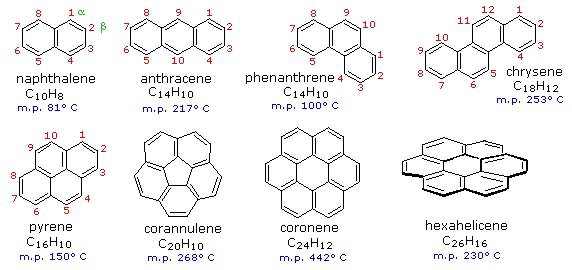
The exception in that case is due to the electrons in the π-bonds. To go back a step, benzene is often drawn with 6 carbon atoms in a ring, with alternating single and double bonds (the Kekulé structure). But actually that is misleading. All 6 bonds are identical. You can consider each of the 6 atoms as sp2 hybridised, i.e. forming 3 σ-bonds at 120deg to one another, 2 to neighbouring carbons and one to hydrogen. That leaves one electron per atom in a p-orbital, perpendicular to the plane of the ring. These p-orbitals overlap with their neighbours to form a delocalised system, all the way round the ring. You can see this in the anomalous magnetic properties of benzene and other so-called aromatic molecules with this type of structure. You can generate a "ring current", causing the electrons to flow round the ring and create a magnetic field. https://en.wikipedia.org/wiki/Aromatic_ring_current The same is true of fused aromatic rings, e.g like the ones in the diagram below: Graphite is what you get if you have an effectively infinite set of these fused rings, forming a sheet (like chickenwire), with successive sheets of rings stacked on top of each other. The bonding between sheets is only weak van der Waals attraction. This is why graphite is slippery (it has applications as a solid lubricant). Regarding conduction of electricity (and heat*) what you end up with is a metal-like situation, with a "sea" of delocalised electrons, able to move across the sheet of rings freely. The difference from a metal is that the electrons are confined to one plane. They cannot easily jump from one sheet to the next. But along the plane of the rings you have metallic behaviour. *Graphite is used for the heat shields of spacecraft, as it conducts heat well along the planes, but very poorly between one and the next. So it is ideal! OLEPS? Wot dey?1 point
-
It's NOT a law, it is a definition. A geometric point is not a physical object. It is an a mental concept that identifies a position and has no other properties.1 point
-
I see light as a wave and never as a particle. Light is quantized for certain but that does not mean it is a particle. Light is emitted and absorbed by electrons in discrete amounts representing the many differences in energy levels from one electron orbital to the next and that is why light waves appear as quanta. The photoelectric effect converts light energy into a stream of electrons but counting electrons thinking that each electron represents a photon is not a valid assumption. BTW it is not "my" discussion or even my OP. I am just another butinski.1 point
-
I'd say he was pretty good a teacher. Still crazy going to Japan then, but how many can say they taught Samurai?1 point
-
I should start by understanding covalent bonds. Firstly there is not one shared electron in a covalent bond but two. Secondly these two electrons do not oscillate from one atom to the other. In truth, once bonded there are no 'atoms' in a covalently bonded molecule.1 point
-
A changing magnetic field will classically induce an EMF. If a conductor is available, a current may then flow. But in the case of an electron in a molecular orbital, (i) this does not produce a changing magnetic field and (ii), as I've pointed out, there is no conductor if the bonding is covalent.1 point
-
Permanent magnets do not rely on current flow to create a magnetic field. The magnetic moment of an electron arises from its intrinsic angular momentum (i.e. spin), as exchemist has already noted1 point
-
No it is not subject specific and my answer is not subject specific either. Self study is wonderful and the higher the level you go the more you have to study that way rahter than a course structured by others. However in my view the real difference between school and self study is that school (should) offers marked work or work guidance. That is the opportunity to get soemthing wrong and then to discuss with your teacher how and why you got it wrong and then to correct it. Or even just the simple yeah you did those well 10/10. With self study most people cannot do this for themselves. Coming to a forum such as this one is a great way to extend the base you are gaining from schoolwork by discussion with others here. If you are lucky you can also have great discussions with your classmates. It is often said that you can learn almost as much from your classmates as your teachers, I certainly did and was lucky that way. So get the best you can out of school and extend it outside.1 point
-
This is about accumulated human knowledge, and learning it in the proper order to make YOUR best use of it. A successful curriculum is what schools offer students, and access to immediate human resources when there's something you don't understand. Think of school and what you learn there as your "toolbox", the basic information you need to make informed decisions. And self-learning is great too! Using other sources (Khan Academy leaps to mind, always) on top of what you're learning in school is a great way to challenge yourself and find areas of interest in your studies. We've had other threads about online study if you want to do a Search. Discussing specifics about a subject with other interested folks is a great way to learn as well. We specialize in science discussion here, and I would encourage you to ask questions if there's anything you want more knowledge about. The members here are amazing in their diverse knowledge, and more than willing to help everyone involved in the conversations sharpen their reasoning tools.1 point
-
Apart from what @Ghideon said: You have missing semicolons in several places. Groovy online interpreter accepts it, but it will learn you bad habits. 'slope' and 'intercept' are nowhere declared and then used as either variable and as method/function name and also nowhere declared.. My fixed version which passes through online Groovy interpreter: https://www.tutorialspoint.com/execute_groovy_online.php Looks like: /* Hello World in Groovy */ double slope( double a, double b ) { return( a ); } double intercept( double a, double b ) { return( a ); } double simple_regression(double dependend_values, double independend_values, double x_test) { double slope = slope(dependend_values, independend_values); double intercept = intercept(dependend_values, independend_values); return intercept + slope * x_test; } But you will need to fill slope() and intercept() with proper functionality. SimpleRegression java class from: https://commons.apache.org/proper/commons-math/javadocs/api-3.3/org/apache/commons/math3/stat/regression/SimpleRegression.html doesn't have addData() without parameters..1 point
-
That depends strongly on your definition of 'better'. Without any counterarguments, I see that the race to the top of the 'holier than thou' heap has intensified; there is no need to consider other opinions, and viewpoints, in an echo chamber. Trying to make life better for the oppressed and disadvantaged is a valid and noble cause; appeasing nutbars who want to be 'fashionable' and refer to themselves as 'thou' ( the point Koti was trying to make ), or simply delusional people with mental health issues ( we seem to have growing numbers ) is NOT. Nor is it 'better'.1 point
-
The error indicates that the code is missing an import statement for the class (or package) that defines SimpleRegression. For more information about Java import ser for instance : https://www.w3schools.com/java/ref_keyword_import.asp1 point
-
The philosophy seems to be nihilism as interpreted by the most self-loathing. With a paradox at the center: If adherents self-apply the core doctrine, then they may well increase the suffering of family and friends, which would then contradict their stated goal. If they destroy animals and forests, then they increase human suffering, which again contradicts their goal. Any philosophy that can only be successfully implemented by a total holocaust is not worth your time.1 point
-
0 points
-
If my attitude offends you that is a you problem. questions ive already asked. the world is surely diverse and my "attitude" is a part of that tapestry , either you can swallow that or not. not my problem. in the words of val kilmer in tombstone Why Ed does this mean we're not friends anymore? You know Ed, if I thought you weren't my friend... I just don't think I could bear it!”-1 points